Bradycardias: Sinus Nodal Dysfunction and Atrioventricular Conduction Disturbances
Deborah L. Wolbrette
Gerald V. Naccarelli
Overview
Sinus nodal dysfunction and atrioventricular (AV) block account for the majority of significant bradyarrhythmias. In addition to structural abnormalities, drug effects and autonomic influences can cause sinus nodal dysfunction. Acquired AV block is most commonly caused by idiopathic fibrosis, acute myocardial infarction, or drug effects. Patients with asymptomatic sinus bradycardia or sinus pauses have a good prognosis and do not require treatment. On the other hand, those with tachycardia-bradycardia syndrome have a much worse prognosis because of their risk of thromboembolic complications. Therefore, the aim of therapy is prevention of atrial fibrillation. Atrial pacing and anticoagulation can greatly reduce the incidence of stroke in this high-risk group. Once appropriate pacing has been established for AV block, the prognosis is primarily dependent on the extent of the associated heart disease.
Sinus Nodal Dysfunction
Anatomy and Physiology of the Sinus Node
The normal heartbeat arises from the sinus node, which is located laterally in the epicardial groove of the sulcus terminalis, near the junction of the superior vena cava and right atrium. The sinus node comprises of “nests” of principal pacemaker cells, which spontaneously depolarize and are situated within a fibrous tissue matrix. However, instead of a discreet point of impulse initiation, the sinus node is in reality a “region” (1). In addition to the nest of principal pacemaker cells, other nests contain cells with slower intrinsic depolarization rates and serve as backup pacemakers in response to changing physiologic and pathologic conditions. Therefore, the principal pacemaker site shifts within the sinus nodal region, resulting in subtle changes in P-wave morphology (2). Although blood supply to this region predominantly comes from the sinus nodal artery, which is a branch of the right coronary artery in 65% of patients, the variability of coronary anatomy and blood supply to the region makes the sinus node more vulnerable to damage during operative procedures (3).
Normal conduction velocities within the sinus node are slow, on the order of 2 to 5 cm/sec, increasing the likelihood of intranodal conduction block (4). Both parasympathetic and sympathetic mediators influence the rate of spontaneous depolarization in pacemaker cells and may cause a shift in the principal pacemaker site within the sinus nodal region (4,5). Acetylcholine, from parasympathetic nerve endings, reduces the rate of cell depolarization and prolongs cell refractoriness. Therefore, increased parasympathetic activity can produce sinus bradycardia, sinus arrest, and sinoatrial exit blocks (6). On the other hand, sympathetic mediators, such as norepinephrine and epinephrine, can increase the sinus rate and reverse sinus arrest and sinoatrial exit block (7).
Electrocardiographic Features of Sinus Nodal Dysfunction
Inappropriate Sinus Bradycardia
Sinus bradycardia (sinus rate <60 beats per minute) is considered inappropriate when it is persistent and does not increase appropriately with exercise. This arrhythmia should be distinguished from asymptomatic resting sinus bradycardia in young athletes and in normal adults during sleep (8,9). Chronotropic
incompetence is not present in these individuals, as it is in patients with sinus nodal dysfunction.
incompetence is not present in these individuals, as it is in patients with sinus nodal dysfunction.
Sinus Arrest
The terms sinus arrest and sinus pause are used interchangeably and refer to the condition in which the sinus node’s principal pacemaker cells fail to fire. The pause is not an exact multiple of the preceding PP interval. Pauses greater than 3 seconds are rare in normal individuals and may or may not be associated with symptoms but are usually caused by sinus nodal dysfunction (10,11). In contrast, asymptomatic pauses greater than 2 seconds (but <3 seconds) are seen in 11% of normal patients during 24-hour Holter monitoring and are especially common in trained athletes (12).
Chronic Atrial Fibrillation
The presence of chronic atrial fibrillation in a patient with a slow ventricular response not secondary to drug therapy is a sign of sinus nodal dysfunction. In some cases, cardioversion results in a long sinus pause before the appearance of sinus rhythm, or junctional escape rhythm. Although a combination of sinus nodal and AV nodal conduction disease may be present in many instances, examples of rapid ventricular responses during atrial tachyarrhythmias can frequently be found.
Tachycardia-Bradycardia Syndrome
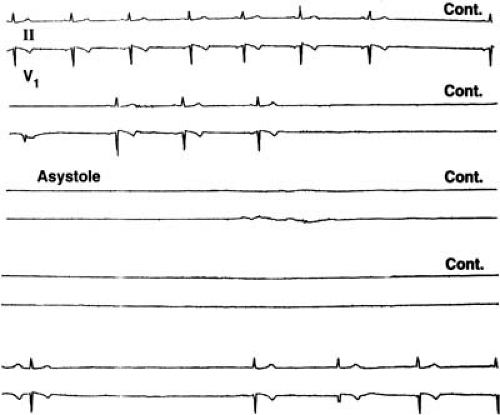
Tachycardia-bradycardia syndrome refers to the presence of intermittent sinus or junctional bradycardia alternating with atrial tachycardia (usually paroxysmal atrial fibrillation) in the same patient. This condition, frequently referred to as sick sinus syndrome, is a common manifestation of sinus nodal dysfunction. The highest incidence of syncope associated with sinus nodal dysfunction probably occurs in this group. Syncope typically occurs secondary to a long sinus pause after the spontaneous termination of atrial fibrillation (Fig. 62.1).
Pathophysiology
Intrinsic Sinus Nodal Dysfunction
Idiopathic degenerative disease is probably the most common cause of intrinsic sinus nodal dysfunction. Fibrous tissue replacement with age explains some but not all cases (13,14). Coronary artery disease may be responsible for one third of cases of sinus nodal dysfunction (15). Transient slowing of the sinus rate, or sinus arrest, can complicate an acute myocardial infarction. This is usually seen with an acute inferior wall myocardial infarction, is caused by neural influences, and rarely persists (16). Cardiomyopathy, long-standing hypertension, infiltrative disorders, collagen vascular diseases, inflammatory processes, orthotopic cardiac transplantation, myotonic dystrophy or Friedreich ataxia, and surgical trauma can also result in sinus nodal dysfunction (17,18).
Extrinsic Sinus Nodal Dysfunction
In the absence of structural abnormalities, the predominant causes of sinus nodal dysfunction are drug effects and autonomic influences. Drugs known to depress sinus nodal function include β-blockers, calcium channel blockers, digoxin (22), and sympatholytic antihypertensives (e.g., clonidine, methyldopa, and reserpine) (23). Types IA, IC, and III antiarrhythmic drugs can depress sinus nodal function (22) and occasionally may produce proarrhythmias in patients with sick sinus syndrome. Paroxysmal primary atrial tachycardias and pause-dependent ventricular tachycardia have been described (24,25). Other drugs reported to affect sinus nodal function include lithium, cimetidine, amitriptyline, and phenytoin (22).
Sinus nodal dysfunction may sometimes result from excessive vagal tone in individuals without intrinsic sinus nodal disease. Autonomically induced asystole has been reported to occur rarely and may result in sudden death (26). Hypervagatonia can be seen in carotid sinus syndrome and vasovagal syncope (Fig. 62.2). Well-trained athletes with increased vagal tone may require some deconditioning to help prevent symptomatic bradyarrhythmias (27).
Clinical Profile
Incidence and Symptoms
The estimated incidence of sinus nodal dysfunction in patients older than 50 years of age is 3 in 5,000 (28). Syncope and presyncope are the most frequent symptoms associated with significant bradycardia. Fatigue, angina, and shortness of
breath can also be seen. Elderly patients may have more subtle symptoms of gastrointestinal distress or change in mental status. Patients with tachycardia-bradycardia syndrome may only experience palpitations associated with tachycardia or embolic events. Syncope in an individual with paroxysmal atrial fibrillation is classically associated with a long sinus pause at the spontaneous termination of the tachycardia. The intermittent nature of these symptoms makes documentation of the associated rhythm disturbance difficult at times. In other cases, marked sinus bradycardia and sinus pauses may be asymptomatic (29).
breath can also be seen. Elderly patients may have more subtle symptoms of gastrointestinal distress or change in mental status. Patients with tachycardia-bradycardia syndrome may only experience palpitations associated with tachycardia or embolic events. Syncope in an individual with paroxysmal atrial fibrillation is classically associated with a long sinus pause at the spontaneous termination of the tachycardia. The intermittent nature of these symptoms makes documentation of the associated rhythm disturbance difficult at times. In other cases, marked sinus bradycardia and sinus pauses may be asymptomatic (29).
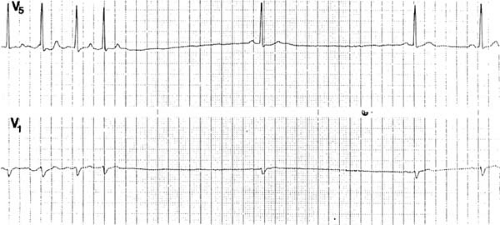 FIGURE 62.1. These rhythm strips of leads V1 and V5 were recorded simultaneously and depict sinus pauses of 3.0 and 2.8 seconds posttermination of atrial tachycardia. |
Diagnostic Techniques
Both noninvasive and invasive means of diagnosing sinus nodal dysfunction are available. Generally, the noninvasive methods of electrocardiographic (ECG) monitoring, exercise testing, and autonomic testing are used first. However, if symptoms are infrequent, invasive electrophysiologic testing or an implantable monitor may be required.
Noninvasive Testing
A 12-lead ECG correlating bradycardia during syncope or near-syncopal episodes can be diagnostic. However, the diagnosis of sinus nodal dysfunction as the etiology of these symptoms is rarely made from the simple ECG. If symptoms are frequent, 24- or 48-hour ambulatory Holter monitoring can be useful. Documentation of symptoms in a diary by the patient while wearing the Holter monitor is essential for correlation of symptoms with the heart rhythm at the time. Often the sinus pauses recorded are not associated with symptoms. Several Holter monitor studies (11,30) demonstrated the futility of treating asymptomatic pauses, even if they were 3 seconds or longer. Most pauses, ranging from 2 to 15 seconds, were asymptomatic, and pacing did not benefit those without associated symptoms. The length of the pause correlated poorly with symptoms and prognosis. In patients whose symptoms occur infrequently, a loop recorder or a home recording device such as Cardionet is useful. Exercise testing is of limited value in diagnosing sinus nodal dysfunction. However, in some cases, it is useful in differentiating those patients with chronotropic incompetence from patients with resting bradycardia who demonstrate a normal heart rate increase with exercise.
Autonomic testing of the sinus node includes various pharmacologic interventions and maneuvers to test reflex responses. An abnormal response to carotid sinus massage (pause >3 seconds) may indicate sinus nodal dysfunction, but this response may also occur in asymptomatic elderly individuals (31). The most commonly used pharmacologic intervention is used to determine the intrinsic heart rate. Complete autonomic blockade is accomplished by administering atropine, 0.04 mg/kg, and propranolol, 0.20 mg/kg. The resulting intrinsic heart rate represents the sinus nodal rate without autonomic influences. The normal intrinsic heart rate is age dependent and can be calculated using the following equation: intrinsic heart rate (in beats per minute) = 118.1 – (0.57 × age [in years]) (32). A low intrinsic heart rate is consistent with abnormal intrinsic sinus nodal function. A normal intrinsic heart rate in a patient with known sinus nodal dysfunction suggests abnormal autonomic regulation.
Invasive Testing
Sinus nodal function can also be evaluated invasively with an electrophysiologic study. This is usually reserved for symptomatic patients in whom sinus nodal dysfunction is suspected but cannot be documented in association with symptoms by noninvasive means. The pacing tests most commonly used are sinus nodal recovery time and sinoatrial conduction time (33). However, even when they are performed appropriately, the ability of these tests to provide a definitive diagnosis is limited (34). The reader is referred to Chapter 62 for further information on sinus nodal function tests.
Natural History
The natural history of sinus nodal dysfunction depends largely on the type of dysfunction and the presence of concomitant cardiovascular disease. The worst prognosis is associated with the tachycardia-bradycardia syndrome. Because stroke is associated with atrial fibrillation, patients with sinus nodal dysfunction complicated by chronic or intermittent atrial fibrillation have an increased risk of embolization.
Literature reviews (35,36) have revealed a significantly lower incidence of atrial fibrillation and thromboemboembolic events in atrially paced patients compared with those only ventricularly paced. In the future, increased use of atrial pacing and anticoagulation therapy should significantly reduce the incidence of stroke in high-risk patients with tachycardia-bradycardia syndrome. Concomitant AV nodal disease is present in about 17% of patients with sinus nodal dysfunction, and new AV conduction abnormalities develop at a rate of about 2.7%/year (35).
Principles of Management
For the patient with asymptomatic bradycardia or pauses and no atrial fibrillation, no treatment is necessary. In the case of symptomatic patients with sinus nodal dysfunction and atrial fibrillation, therapy depends on whether the symptoms are related to the tachycardia or bradycardia episodes. Depending on the etiology of the symptoms, drug therapy may be needed to control rapid ventricular response, pacing may be advised to prevent bradycardia, or both treatment modalities may be required. Ideally, if a patient with symptomatic bradycardia is on a drug known to depress sinus nodal function, the drug should be stopped, or permanent pacing may be required to allow continued use of the needed drug.
Sinus nodal dysfunction is the most commonly reported diagnosis for pacemaker implantation. However, once the
decision to pace is made, choosing the optimal pacemaker prescription is essential to decrease stroke risk and improve quality of life. Atrial pacing has been shown to greatly decrease the incidence of atrial fibrillation and thromboembolism in this population, whereas patients who are ventricularly paced have not seen a similar benefit (35). Therefore, the majority of patients with symptomatic sinus nodal dysfunction should receive a dual-chamber pacemaker. Single-chamber ventricular pacing is reserved for use in patients with chronic atrial fibrillation. For patients with sinus nodal dysfunction who have normal AV conduction, a single-chamber atrial pacemaker is a reasonable choice. Rate-adaptive pacing and mode switching are important for most patients with sinus nodal dysfunction, and are generally standard features in currently available pacemakers. Guidelines for implantation of pacemakers in patients with sinus nodal dysfunction have been established by a task force from the American College of Cardiology and American Heart Association (37). Because of the high risk of thromboembolic events in patients with sinus nodal dysfunction and atrial fibrillation, anticoagulation with warfarin should be considered in these patients.
decision to pace is made, choosing the optimal pacemaker prescription is essential to decrease stroke risk and improve quality of life. Atrial pacing has been shown to greatly decrease the incidence of atrial fibrillation and thromboembolism in this population, whereas patients who are ventricularly paced have not seen a similar benefit (35). Therefore, the majority of patients with symptomatic sinus nodal dysfunction should receive a dual-chamber pacemaker. Single-chamber ventricular pacing is reserved for use in patients with chronic atrial fibrillation. For patients with sinus nodal dysfunction who have normal AV conduction, a single-chamber atrial pacemaker is a reasonable choice. Rate-adaptive pacing and mode switching are important for most patients with sinus nodal dysfunction, and are generally standard features in currently available pacemakers. Guidelines for implantation of pacemakers in patients with sinus nodal dysfunction have been established by a task force from the American College of Cardiology and American Heart Association (37). Because of the high risk of thromboembolic events in patients with sinus nodal dysfunction and atrial fibrillation, anticoagulation with warfarin should be considered in these patients.
Atrioventricular Nodal Dysfunction
Anatomic Considerations
The AV node lies directly above the insertion of the septal leaflet of the tricuspid valve and anterior to the ostium of the coronary sinus. It is part of the AV junction area, which is divided into three regions. The transitional cells, or nodal approaches, connect the atrial myocardium to the compact portion of the AV node. At its distal end, the compact portion of the AV node enters the central fibrous body, becoming the penetrating portion, or His bundle (38). The slowest conduction time (0.03 m/second) occurs within the AV node (39). The blood supply to the AV node is via the AV nodal artery, a branch of the right coronary artery in 90% of hearts, with the remaining 10% arising from the circumflex artery (40). The His bundle has a dual blood supply from branches of the anterior and posterior descending coronary arteries (41).
Like the sinus node, the AV node is richly innervated, and both are influenced by the right and left vagus nerves and stellate ganglia. However, stimulation of the nerves on the right has less affect on AV nodal conduction than on sinus rate. Conversely, left-sided autonomic stimulation exerts stronger influence on AV nodal conduction than on sinus rate (42,43).
Electrocardiographic and Electrophysiologic Findings
Normal Atrioventricular Conduction
On the surface ECG, the PR interval is normally between 120 and 200 msec in duration. This interval reflects the conduction time from the high right atrium to the point of ventricular activation. To measure the different components of the conduction system that the PR interval includes, intracardiac tracings from the high right atrium and bundle of His region are needed. The PA interval, measured from the high right atrial electrogram to the low right atrial deflection in the bundle of His electrogram, gives an indirect approximation of the right atrial conduction time. The AH interval, measured from the low atrial to the bundle of His deflection, reflects conduction time through the AV node. The HV interval, measured from the bundle of His deflection to the earliest ventricular depolarization on the surface electrogram, represents the conduction time from the proximal bundle of His to the ventricular myocardium (44) (Table 62.1).
TABLE 62.1 Normal Conduction Intervals in Adults | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
First-Degree Atrioventricular Block
First-degree AV block on the surface ECG is seen as a PR interval greater than 0.20 second. Each P wave is followed by a QRS complex with a constant, prolonged interval. Although the conduction delay can be anywhere along the system, the PR prolongation is usually caused by delay within the AV node (87% when the QRS complex is narrow). On the bundle of His electrogram, this would be seen as an AH interval greater than 130 msec with a normal HV interval. In cases in which first-degree AV block is seen in the presence of a bundle branch block, a bundle of His electrogram is necessary to localize the site of block (45,46,47). Infranodal conduction delay is present in 45% of these cases. A combination of delay within the AV node and in the His-Purkinje system must also be considered (48). In certain cases of congenital structural heart disease, such as Ebstein anomaly of the tricuspid valve or endocardial cushion defects, intraatrial conduction delay can cause first-degree AV block. In addition, intra-Hisian conduction delay can cause first-degree AV block. On the bundle of His electrogram, a split His potential can be seen, resulting in a prolonged His potential, HV, and PR intervals (52). Dual-AV-nodal physiology can produce transient, abrupt, or alternating first-degree block caused by block in the fast AV nodal pathway (which is normally used), with conduction down the slow pathway instead.
Second-Degree Atrioventricular Block
Type I
Type I second-degree AV block, or Wenckebach block, manifests on the surface electrogram as progressive prolongation of the PR interval before failure of an atrial impulse to be conducted to the ventricles. The PR interval immediately postblock returns to its baseline interval, and the sequence begins again. Features of typical Wenckebach periodicity include the following:
Progressive lengthening of the PR interval throughout the Wenckebach cycle.
Lengthening of the RR interval occurring at progressively decreasing increments, resulting in progressive shortening of the RR intervals.
A pause including the nonconducted P wave that is less than the sum of any two consecutively conducted beats.
Shortening of the PR interval postblock compared with the PR interval just preceding the blocked cycle.
Wenckebach block is almost always within the AV node when a narrow QRS complex is present (49). Intra-Hisian block is the rare exception (50). When type I block is seen with a bundle branch block, the block is still more likely to be in the AV node, but it could also be localized below the bundle of His. A bundle of His electrogram would be needed to accurately identify the level of block. Wenckebach block in the AV
node is characterized by progressive prolongation of the AH interval until an atrial deflection is not followed by a bundle of His or ventricular deflection. In type I block secondary to block below the bundle of His, progressive prolongation of the HV interval is followed by an H deflection without an associated ventricular depolarization.
node is characterized by progressive prolongation of the AH interval until an atrial deflection is not followed by a bundle of His or ventricular deflection. In type I block secondary to block below the bundle of His, progressive prolongation of the HV interval is followed by an H deflection without an associated ventricular depolarization.
Type II
Type II, or Mobitz II, second-degree AV block is characterized on the surface electrogram by a constant PR interval followed by sudden failure of a P wave to be conducted to the ventricles. The PP intervals remain constant and the pause including the blocked P wave equals two PP intervals. Therefore, Mobitz II block should not be confused with a nonconducted premature atrial complex. Mobitz II block is usually associated with bundle branch block or bifascicular block. In a majority of these cases, the site of block is within or below the bundle of His (Fig. 62.3) (46,48). When presumed Mobitz II block is seen in conjunction with a narrow QRS complex, Mobitz I with only minimal PR variation should be suspected. Only rarely is Mobitz II found with a narrow QRS complex and is caused by intra-Hisian block (51). The bundle of His electrogram is useful in verifying the site of the Mobitz II block. The blocked cycle features atrial and bundle of His deflections without a ventricular depolarization. The conducted beats usually show evidence of infranodal conduction system disease, with a prolonged HV interval, or even a split bundle of His potential (48).
2:1 Atrioventricular Block
Fixed 2:1 AV block poses a diagnostic dilemma because it is usually impossible to classify as type I or II block by a surface electrogram alone. A narrow QRS complex and recently seen Wenckebach block is highly suggestive of block at the AV nodal level. A 2:1 block associated with a wide QRS complex is likely infranodal, but it could still be at the level of the AV node. A definitive diagnosis can only be made with an intracardiac recording at the bundle of His region.
Nonconduction of two or more consecutive P waves when AV synchrony is otherwise maintained is sometimes termed high-degree AV block. The level of block can be at the AV node or the His-Purkinje system. When high-degree AV block is caused by block in the AV node, QRS complexes of the conducted beats are usually narrow. Wenckebach periodicity is also seen, and atropine administration produces 1:1 conduction. Features pointing toward block in the His-Purkinje system are conducted beats with bundle branch block and no improvement in block with atropine. Bundle of His recordings are sometimes needed to confirm the site of block.
Third-Degree Atrioventricular Block
Third-degree, or complete, AV block is seen on the surface electrogram as completely dissociated P waves and QRS complexes, each firing at its own pacemaker rate. The atrial impulse is never conducted to the ventricles, but different levels of block are possible. The level of block determines the QRS morphology along with the site and rate of the escape rhythm. Congenital complete heart block is characterized by a narrow QRS complex with an escape rate between 40 and 60 beats per minute, which tends to increase with exercise or atropine. This is consistent with block within the AV node. Acquired complete heart block is usually associated with block in the His-Purkinje system, resulting in a wide QRS complex with an escape rate between 20 and 40 beats per minute. The intracardiac electrogram shows bundle of His deflections consistently following the atrial electrograms, but the ventricular
depolarization is completely dissociated from these. Block below the bundle of His is thus demonstrated. In contrast, complete heart block at the AV nodal level is seen on the intracardiac tracings as bundle of His potentials consistently preceding each ventricular depolarization. The atrial electrograms are dissociated from the HV complexes (Fig. 62.4). The sinus rate is faster than the ventricular rate in patients with complete heart block. Data collected from patients with congenital complete heart block have shown the atrial rate to usually be age appropriate (52). It is important to note that complete antegrade AV block does not always predict retrograde (VA) conduction. Retrograde conduction may be intact in an individual with complete antegrade AV block (Fig. 62.5).
depolarization is completely dissociated from these. Block below the bundle of His is thus demonstrated. In contrast, complete heart block at the AV nodal level is seen on the intracardiac tracings as bundle of His potentials consistently preceding each ventricular depolarization. The atrial electrograms are dissociated from the HV complexes (Fig. 62.4). The sinus rate is faster than the ventricular rate in patients with complete heart block. Data collected from patients with congenital complete heart block have shown the atrial rate to usually be age appropriate (52). It is important to note that complete antegrade AV block does not always predict retrograde (VA) conduction. Retrograde conduction may be intact in an individual with complete antegrade AV block (Fig. 62.5).
 Get Clinical Tree app for offline access 
|