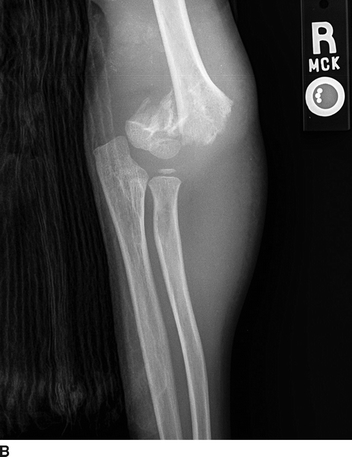
FIGURE 1 A, B:Radiographic images of a Gartland type III fracture of the right supracondylar humerus (Case vignette 1).
Differential Diagnosis
A fractured humerus should always lead to a clinical suspicion of a brachial artery injury. Although the artery is injured in a minority of these types of fractures, the proximity of the vessel to the humerus and the harmful consequences of a missed vascular injury call for an astute clinical evaluation. One may encounter two categories of humerus fracture, open and closed. In both of these categories of fracture, the likelihood of brachial artery injury is proportional to the severity of the fracture including the degree of displacement or malalignment. Closed fractures are most common after a fall such as in the first case vignette or other forms of blunt trauma. In contrast, open fractures result from penetrating mechanisms such as gunshot or explosive events. In the most severe of injuries, a blunt humerus fracture may result in the bone penetrating through the skin, which is also considered an open fracture. The differential diagnosis of patients with an injured upper extremity with arm and hand symptoms includes brachial artery injury with varying degrees of ischemia, compression or entrapment of the main peripheral nerves coursing distally through the extremity (radial, ulnar, or median), and/or pain from the fracture.
Workup
Physical Examination
As one examines the patient with an injured upper extremity, it is useful to consider the differential diagnosis, which, in addition to fracture pain fracture, includes brachial artery spasm, peripheral nerve (i.e., median, radial and ulnar) entrapment, or brachial artery injury with varying degrees of ischemia. In the presence of a humerus fracture, spasm of the brachial artery may result in an initially abnormal-appearing arm and hand with reduced or absent pulses or Doppler signals. This phenomenon is especially common in younger patients, and the secondary vasospasm will often resolve with return of pulses and Doppler signals after reduction and alignment of the fracture. Similarly, after fracture reduction, any artery or nerve entrapment will often resolve with improvement of more distal signs and symptoms.
The physical examination of patients with a suspected or obvious humerus fracture includes assessing the extremity for deformity and a careful evaluation of perfusion to the hand and fingers. Depending on the setting, the physical exam can be performed simultaneous with plain radiographs (multiple views of the humerus, the shoulder, and the elbow) to diagnose and assess the severity of the fracture (Fig. 1A and B). A general guide is that a posterior or lateral fracture dislocation of the humerus is more commonly associated with a brachial artery injury, whereas a medial or anterior dislocation is more commonly associated with radial nerve injury.
The vascular exam of the upper extremity begins with observing for hard signs of vascular injury such as arterial bleeding from open wound(s), an expanding or tense hematoma, or obvious ischemia of the hand and fingers. Palpation for the brachial pulse just above and medial to the antecubital fossa and the radial and ulnar pulses at the wrist should be performed, and capillary refill should be estimated on the distal aspect of the fingers.
Continuous wave Doppler is an extension of the physical exam in patients with a humerus fracture. Doppler signals should be assessed in the same locations at which pulses were palpated (antecubital fossa, radial and ulnar arteries at the wrist). Strong, biphasic signals audible at these locations argues against a brachial artery injury, while weak, monophasic, or absent arterial signals signify a flow-limiting arterial defect. To more objectively assess perfusion of the arm and hand, one can measure the IEI.
The IEI is a ratio of the occlusion pressure of the distal arterial Doppler signal in the injured compared to the noninjured upper extremity. To accomplish this, a manual blood pressure cuff is placed over the forearm of the injured upper extremity and inflated while the Doppler signal is being listened to at the radial and then the ulnar artery. The cuff pressure at which the arterial signal occludes at each of the arteries should be recorded and then these steps repeated on the uninjured upper extremity. The IEI is calculated by dividing the highest occlusion pressure on the injured extremity by the occlusion pressure of the uninjured extremity with a normal index being 0.9 or greater. The IEI provides an objective and repeatable measure by which to assess for arterial injury and is especially useful in scenarios where perfusion distal to the humerus fracture may not be obvious.
Additional Imaging versus Operation
Patients with hard signs of brachial artery injury (i.e., external hemorrhage, profound or obvious ischemia, arteriovenous fistula, and expanding hematoma) typically do not need additional imaging or workup prior to being taken to the operating room for exploration and treatment. One exception to this is the case in which the patient has additional injuries that require further imaging. For example, the patient in the second case vignette had the hard sign of hemorrhage requiring tourniquet application and requires little additional imaging of the arm before the operation. However, this patient may very well require imaging of other injuries (i.e., head, chest, and abdomen) prior to initiating treatment of the arm.
Patients who have a humerus fracture and soft or less clear signs of vascular injury (i.e., modestly reduce IEI, audible but abnormal sounding Doppler signals, equivocal capillary refill) pose a more challenging diagnostic scenario. In the first clinical vignette, additional imaging with arteriography, contrast tomography (CT), or duplex ultrasonography may be useful in making the decision as to whether or not to operate on the brachial artery. Because of the relatively superficial nature of the brachial artery, duplex ultrasound is often the imaging modality of choice as it is quick, inexpensive, and noninvasive. However, standard catheter-based arteriography or CT angiography may also be used to discern the presence of flow-limiting defects or injuries in the brachial artery following humerus fracture.
It is important to restate that in all cases of humerus fracture that the initial step of management, often even before additional imaging, is fracture reduction and stabilization of the extremity. This can often be performed outside of the operating room following hemorrhage control, initiation of resuscitation, and pain control measures. In nearly all cases of significant fracture with dislocation or misalignment, this management step should be pursued regardless of initial signs of vascular injury. Depending on the severity of and the time since the injury, reducing and stabilizing the fracture may favorably change perfusion to the arm and the associated vascular exam. In most cases of mild to moderate humerus fracture, the decision as to whether or not to operate on or further image a brachial artery is made after the fracture is reduced and the vascular exam is repeated. If signs of vascular injury persist after fracture reduction and stabilization, then additional imaging with one of the previously noted modalities is indicated. In cases of extreme open humerus fracture (i.e., the mangled extremity) such as the second case vignette, fracture reduction and stabilization and exploration of the brachial artery should occur simultaneously in the operating room.
Treatment Decision
If physical signs of malperfusion persist or evidence of brachial artery injury are evident after fracture reduction, efforts to restore normal perfusion to the arm and hand should be undertaken in most cases. In rare instances of damage control surgery or those cases in which the ischemia is incomplete (i.e., injury distal to the profunda brachii) and felt to be tolerable, brachial artery injuries can be observed. However, like lower extremity ischemia, chronic malperfusion to the arm and hand can lead to debilitating neuromuscular atrophy and dysfunction and even tissue loss.
Surgical Approach
Brachial artery injury should be approached with an open surgical incision in nearly all cases. While isolated reports of catheter-based treatment of this injury pattern exist, the brachial artery’s superficial and accessible position in the upper extremity and its relatively small size make it most amenable to open repair. To expose and operate on the brachial artery, the patient should be placed supine on the operating table with the arm extended out onto an arm table. To facilitate concurrent management of the humerus fracture, it is often useful to have a radiolucent arm board and mobile fluoroscopy.
The axilla should be shaved and the hand and fingers included in the surgical scrub so that they are accessible to examine during and after revascularization. The brachial artery should be approached through a median incision in the upper arm in the crease between the biceps and triceps muscle groups. The incision should be positioned proximal enough to allow exposure and control of the brachial artery, which can include extending the exposure proximal into the axilla through an S-shaped incision if needed.
The brachial artery rests in the groove between the biceps and triceps muscle groups and is adjacent and often crossed by one or more brachial veins. From its proximal segment, the brachial artery gives rise to a deep arterial branch referred to as the profunda brachii. Depending on its size, this artery can provide a significant amount of perfusion to the distal upper extremity assuming it or its collateral branches have not been injured with the fracture. In some instances in which the extent of the profunda brachii is preserved, ligation of the main brachial artery distal to the profunda origin is well tolerated. If this approach is considered, it will be necessary to monitor distal perfusion in the intra- and perioperative period to assure that the deep brachial artery is sufficient to avoid adverse effects of ischemia.
The median nerve courses adjacent to the brachial artery and veins through most of the upper arm, and some have referred to this relatively fixed compartment as the axillary and brachial sheath. Once the area of brachial artery injury is exposed, proximal and control of the vessel with loops or small vascular clamps should be obtained. The zone of brachial artery injury should be dissected free of surrounding structures and the extent of injury determined. Depending on the severity of injury, vascular repair will require primary closure, patch angioplasty, or interposition grafting. Because of its relatively small and elastic nature, most significant brachial artery repairs will require removal of the injured segment and restoration of flow using an interposition graft.
Prior to repairing the brachial artery, removal of thrombus proximal and distal to the injury should be accomplished using a small (2 or 3 French) Fogarty thromboembolectomy catheter. Once this has been accomplished, one should assure that there is ample fore and back bleeding and then instill heparinized saline solution proximal and distal to the injured segment. The edges of the injured vessel should be debrided to assure that the suture repair is performed through normal or nontraumatized, full-thickness vascular wall. Regardless of the type of reconstruction (primary, patch, or interposition), repair should be completed using fine (6-0 or 7-0), monofilament, permanent suture such as polypropylene (Prolene). In most cases, autologous saphenous vein should be used for the patch or the interposition graft. If vein is not available, synthetic path or conduit such as ePTFE (expanded polytetrafluoroethylene) may be used. Assessment of perfusion following completion of the vascular repair can be made using intraoperative Doppler or duplex and occasionally contrast arteriography. The vascular repair should be covered with viable muscle and soft tissue to reduce the likelihood of anastomotic infection or desiccation and disruption.
Special Considerations for Operative Repair
Temporary Vascular Shunt
The use of temporary vascular shunts as a quicker and simpler way to restore perfusion to the arm and hand in instances of severe brachial artery injury is an accepted damage control maneuver. This adjunct may be especially useful to reduce ischemic times in cases in which the operating team has limited experience with formal vascular reconstruction or in cases where the patient has higher priority, life-threatening injuries. Shunts have also been shown to be effective at quickly restoring perfusion to the arm and hand prior to formal fixation of the humerus fracture. In these cases, the injury is explored, the shunt placed, the fracture reduced and fixated, and then the shunt removed for formal vascular repair.
Use of a temporary vascular shunt for a brachial artery injury should be preceded by the previously described steps including exploration of the injury, proximal and distal control, removal of thrombus, and assurance of proximal inflow and distal back bleeding. At this juncture, the temporary vascular shunt is gently inserted and secured either with the vessel loops or with a silk free tie. Patency of the shunt can be confirmed using continuous wave Doppler. The most commonly used shunts are the smaller (8 or 10 French) Argyl or the shorter (15 cm) Sundt shunt.
Anticoagulation
Heparin sulfate as an anticoagulant should be used when possible in the setting of brachial artery injury and repair. In cases where the injury is isolated and not associated with a significant soft tissue wound, normal or full doses (50 to 75 U/kg) of systemic heparin can be given at the time that the arterial injury is being explored. If the patient has other injuries or a large open, soft tissue wound, which preclude the use of systemic anticoagulation, “regional” heparin can be administered in the form of heparinized saline solution. Typically, heparinized saline contains anywhere between 1000 and 10,000 U of heparin per liter of sterile saline, and this can be flushed proximal and distal to the vascular reconstruction as well as used to irrigate the vessel during the time of the repair. Unless there are untoward events that accompany the vascular repair such as large amounts of distal thrombus or a repair that thromboses and requires revision, the use of heparin in the postoperative period is not required.
Fasciotomy
Fasciotomy is not usually required in the case of brachial artery injury repair because of the relative small mass of muscle in the forearm and hand and the less constrained compartments of the forearm. However, in cases in which the ischemic time has been greater than 6 hours or in which there has been significant blast or crush injury, it is recommended that a fasciotomy be performed. The forearm has three compartments that should be opened at the time of fasciotomy: dorsal or extensor, volar or flexor, and mobile wad. If the volar compartment is tight upon opening, it is recommended that the carpal tunnel be released in order to avoid entrapment or compression of the median nerve.
Postoperative Care and Management
The upper extremity must be closely monitored in the postoperative setting including repeat assessment for adequate perfusion. In most instances, this is performed with a continuous wave Doppler machine and pen although a duplex ultrasound machine is also useful in the mid- and longer-term period. Unless unusual complications accompanied the brachial artery repair, systemic anticoagulation is not required in the postoperative period. In most cases, the heparin from the operating room is allowed to dissipate, and then patients receive low-dose, low molecular weight heparin or antiplatelet therapy or both. If a vascular reconstruction has been performed (primary, patch, or interposition repair), duplex surveillance of this should be performed at 6 and 12 months after the operation. Annual duplex ultrasounds of the repair are desired thereafter although these can be difficult to continue in younger trauma patients.
Case Conclusion
In the first case vignette after initiation of monitored sedation and pain control, the patient has the right supracondylar fracture reduced and aligned. After this maneuver, pulses were able to be palpated in the radial and ulnar arteries. The IEI was repeated and found to be 0.90. The patient underwent duplex ultrasound of the brachial artery at the time of open reduction and internal fixation (ORIF) of the humerus demonstrating no flow-limiting defects. The patient was closely monitored after repair of the fracture including repeat duplex of the brachial artery at 7 and 30 days both of which showed normal flow through the brachial artery.
The patient in the second case vignette was initially treated at a forward surgical team (FST) facility where the tourniquet was removed in the operating room when the penetrating wound was explored. In addition to the open humerus fracture, the brachial artery and median nerve were found to be severed by the penetrating fragment. As a matter of damage control or abbreviated operating, the patient had a shunt placed in the right brachial artery reperfusing the right arm and hand prior to forearm fasciotomy and external fixation of the humerus fracture. At the conclusion of this 60-minute operation, the patient’s arm was stabilized and there were palpable pulses in the radial and ulnar arteries.
The patient was evacuated to a higher echelon of care (level III surgical hospital) where he arrived with a well-perfused hand. A radiograph was taken of his right arm upon arrival demonstrating an aligned humerus fracture and an indwelling temporary vascular shunt (Fig. 2). The patient was taken to the operating room where the right upper extremity wounds were explored and the Javid shunt found to be patent by Doppler exam. The median nerve was identified as severed and tagged with monofilament suture (Fig. 3). The patient was positioned and prepped such that distal great saphenous vein could be harvested and used as conduit for the interposition graft following shunt removal (Fig. Figs. 4 and 5). Following removal of the shunt and placement of a reversed great saphenous vein interposition graft, the patient’s wounds were debrided and irrigated and viable tissue used to close over the vascular reconstruction and severed median nerve. A closed, negative pressure wound therapy device was used to cover the wounds (Fig. 6), and the patient was evacuated out of the theater of war 18 hours later (Table 1).



