Chapter 34 Bone Tumors
Biopsy is a complex cognitive skill in the skeleton. Fine-needle, core, or surgical biopsy tracts harbor malignant cells. Therefore, definitive surgical resection of cancer requires removing the biopsy tract, all iatrogenic contamination, and the bone tumor in an en bloc resection. This requires extensive exposure with wide flaps and mobilization of neurovascular structures. Inappropriately placed biopsy or needle puncture sites can complicate placement of the definitive surgical incision or require multiple incisions, thereby jeopardizing limb salvage. Key structures may be contaminated by the biopsy tract. It has been conclusively shown in several studies that surgeons inexperienced in musculoskeletal oncology principles have a three to four times increased rate of complications from a poorly placed biopsy site.1–3 Unfortunately, this results in unnecessarily complex revision surgery and, in some cases, amputation instead of limb salvage.
Staging of skeletal sarcomas is straightforward and has remained relatively unchanged since its original description by Enneking and colleagues.4 Roman numeral I refers to a low-grade skeletal sarcoma as interpreted by the pathologist. Roman numeral II is high grade. Roman numeral III signifies metastasis, whether regional or distant. The letter A refers to intracompartmental tumor localization, whereas the letter B refers to extracompartmental growth of the primary skeletal sarcoma. A bone tumor that begins in the femur and grows into the quadriceps musculature is extracompartmental because it has grown out of its original compartment into another. Pathologic fractures can be thought of as extracompartmental tumors. The Enneking system has five stages, IA, IB, IIA, IIB, and III. Stage IIB tumors are high risk. Stage III represents metastases of any type. The staging system of the American Joint Committee on Cancer has not been universally adopted for skeletal sarcomas.
Genetics
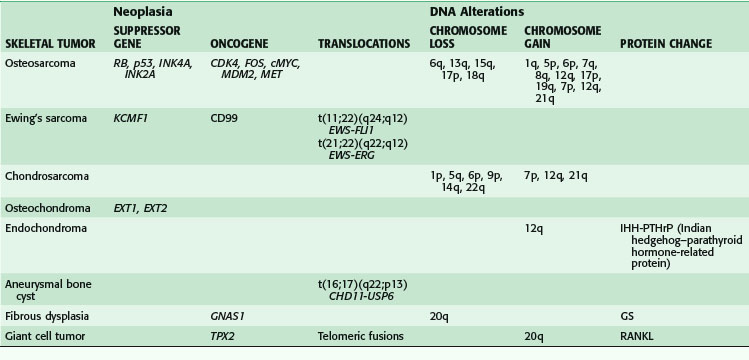
Benign and malignant skeletal neoplasms have a host of DNA alterations catalogued by the absence or presence of suppressor genes, oncogenes, translocations, and chromosome gains or losses. Table 34-1 lists these genetic alterations for some selected bone tumors5; this table is meant to be a summary rather than an exhaustive list.
In contrast, skeletal metastases (e.g., carcinomas) manipulate the normal bone microenvironment to create osteolytic bone destruction while promoting the growth and spread of cancer cells.6,7 Cell adhesion molecules are used for both cell to cell and cell to matrix binding. Deregulation of matrix metalloproteinases disrupts the delicate balance of matrix homeostasis by increasing proteolytic activity. Degradation of the extracellular matrix results in cancer cell invasion. Angiogenesis stimulators such as vascular endothelial growth factor, fibroblast growth factor, and transforming growth factor-β are triggered by cancer cells to promote their own growth. Parathyroid hormone–related protein is released by certain tumor cells acting on the same receptors for parathyroid hormones to promote osteoclast-mediated bone resorption. Osteoclastogenesis is also promoted by interleukin-6, interleukin-8, and RANKL (receptor activator of nuclear factor κB ligand). There is a complex interaction among many cell receptors, cytokines, growth factors, and proteases in the metastatic bone microenvironment.
Benign Bone Tumors
Incidence
The incidence of benign bone tumors far exceeds that of skeletal sarcomas. In our clinical experience, there are at least five benign bone tumors for every primary malignant bone neoplasm. Unni and Inwards8 have found that approximately 54% of benign bone tumors are chondrogenic. Osteochondroma and enchondroma are the most common benign tumors. Both can be polyostotic. Osteochondromas are surface neoplasms of bone, whereas enchondromas are located intraosseously. The true prevalence of these tumors is unknown because many go undetected and unreported.
Surgical Oncology
Reconstruction of benign bone tumors after curettage is often accomplished with a combination of bone grafting and stabilization of impending or completed fractures. Bone grafting can be performed with autogenous bone or allograft. Many allograft preparations are commercially available, including demineralized bone matrix from an American Association of Tissue Banks–approved bone bank.9 Adequate curettage demands a large bone portal to access the intraosseous cavity, which, however, severely compromises the biomechanical integrity of the bone and requires operative stabilization. Stabilization can be done extracorporeally, such as with a cast or splint. Internal bone stabilization can be accomplished with a combination of rods, plates, pins, or screws. The goal is to achieve osteogenesis, preserve skeletal growth, and gain strength within weeks.
Examples
Enchondroma
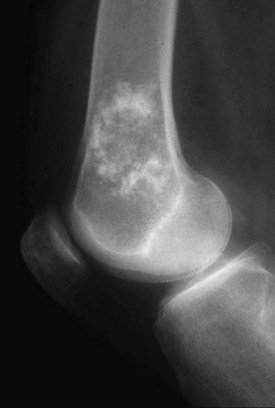
Enchondroma (Fig. 34-1) is a benign proliferation of hyaline cartilage typically found in long bones, but it may also occur in the axial skeleton. Cartilage anlage or islands retain chondroid features and continue to grow until skeletal maturity, when they begin to undergo calcification. Their long-term physiologic activity is why they remain scintigraphically active decades later on a bone scan. An enchondroma typically begins in the metaphysis and extends into the diaphysis. It seldom occurs in the epiphysis of long bones. Polyostotic syndromes may occur, often with unilateral predominance. Ollier’s disease is the eponym associated with multiple skeletal enchondromas. Maffucci’s syndrome is Ollier’s disease associated with multiple subcutaneous hemangiomas. In the pediatric population, management involves maintaining a strong, straight, and symmetrical bone of appropriate length. After skeletal maturity, malignant transformation is rare. However, the greater the tumor burden, the greater the late malignant transformation rate. Therefore, patients with Ollier’s disease often have a higher incidence of chondrosarcoma formation than those with solitary disease. The more axially located tumors of the pelvis, spine, and scapula have the worst prognosis. Interestingly, individuals with Maffucci’s syndrome have the same elevated incidence of chondrosarcoma formation; however, this unique patient population frequently succumbs to the development of occult carcinomas.10
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree