LEARNING OBJECTIVES
Learning Objectives
The student will be able to identify primary acid/base disorders given patient laboratory results and the degree of compensation.
The student will be able to distinguish between the roles of the lungs and kidneys in maintenance of acid/base homeostasis.
The student will be able to identify common causes of metabolic acidosis and alkalosis, and of respiratory acidosis and alkalosis.
The student will be able to use the calculation of the anion gap to identify causes of metabolic acidosis.
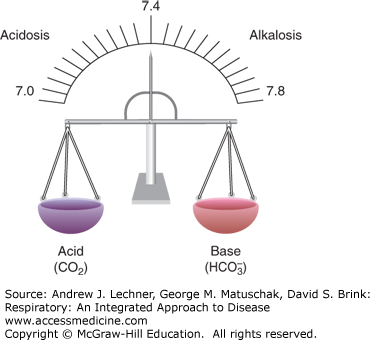
Although the human diet is essentially neutral with respect to its pH, various aerobic and anaerobic metabolic processes create large amounts of acid daily from the catabolism of carbohydrates, fats, and proteins. The body maintains a constant [H+] such that blood and extracellular pH is near 7.4 and intracellular pH is about 7.1. The buffer most responsible for this is the HCO–3/H2CO3 system, regulated by the coordinated behavior of the lungs and kidneys. Aerobic metabolism yields gaseous CO2 that combines with water to form volatile carbonic acid, H2CO3. The lungs are primarily responsible for elimination of CO2. Anaerobic metabolism of glucose and fat, as well as some protein catabolism yield fixed or nonvolatile acids such as lactic acid, sulfuric acid, and phosphoric acid, most being excreted by the kidneys. In one 24-hour period, a 70-kg person produces 70-100 mmol of such nonvolatile acids versus 20 moles CO2, all without appreciable change in plasma pH. An effective V̇A controls CO2 excretion, while the kidneys excrete nonvolatile acids and reabsorb or regenerate HCO–3. The lungs and kidneys maintain a balance of CO2 and HCO–3, and thus pHa near 7.4 (Fig. 17.1).
FIGURE 17.1
Acidosis and alkalosis refer to primary processes that increase or decrease blood [H+], respectively. Acidemia refers to a blood pH <7.36 and alkalemia to a blood pH >7.44. Compensation occurs as either the metabolic (renal) or respiratory response that attempts to restore blood pH when altered by some primary process.
BASIC CATEGORIES OF ACIDOSIS AND ALKALOSIS
Acidosis and alkalosis are terms that describe primary processes modulating [H+] in patient populations. Each may be metabolic or respiratory in origin, based on whether the primary deviation is in arterial [HCO–3] or Paco2, respectively. Respiratory disorders are caused by inappropriate excretion of CO2 that raises or lowers [H2CO3] in body fluids. Metabolic disorders reflect primary changes in plasma [HCO–3] due to excessive intake, production, or loss of HCO–3 or inappropriate handling of H+ and anions from dissociated nonvolatile acids. Primary acid-base disorders and the compensations that must follow to restore pH to normal are shown in Table 17.1.
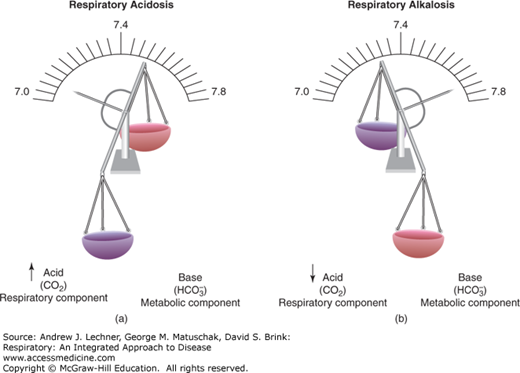
In respiratory acidosis, excessive CO2 raises blood Pco2, and produces by the mass action principle more H2CO3 which then dissociates into H+ and HCO–3 to reduce pHa (Fig. 17.2). Compensation for this drop in pHa is attempted in the kidneys through reabsorption of filtered HCO–3 to restore pHa toward 7.4. In respiratory alkalosis, a reduced Paco2 forces by mass action the resynthesis of H2CO3 from free H+ and HCO–3 and thereby raises pHa (Fig. 17.2). Compensation for the rise in pHa is attempted in the kidneys by excretion of more anion, including HCO–3.
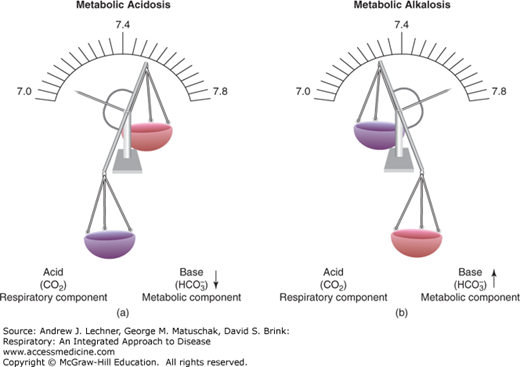
In metabolic acidosis, excess H+ from various nonvolatile acids causes pHa to fall below 7.4, even as blood [HCO–3] decreases due to its recombination with those protons (Fig. 17.3). Respiratory compensation requires hyperventilation to excrete the added acid burden and restore pHa to 7.4, although this response simultaneously lowers Paco2. In metabolic alkalosis, the blood [HCO–3] is abnormally elevated, raising pHa above 7.4. Respiratory compensation attempts to increase Paco2 by hypoventilation, which then acts to restore pHa downward toward 7.4 by forming more H2CO3.
DERIVATION OF THE HENDERSON–HASSELBALCH EQUATION
Laboratory assessment of a patient’s acid-base status involves quantifying components of this HCO–3/H2CO3 buffer system. Arterial blood gas (ABG
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree