CHAPTER 51 Blood Coagulation, Transfusion, and Conservation
After cardiac surgery, patients may exhibit hemostatic abnormalities, both quantitative and qualitative. However, preexisting and acquired problems also contribute to bleeding. The increasing use of antiplatelet agents (e.g., clopidogrel [Plavix]) and anticoagulation agents contributes to a compromised preoperative hemostatic state that is exacerbated by the tissue injury associated with surgery.1 This complex hemostatic alteration is further complicated by heparin and cardiopulmonary bypass (CPB), which result in additional acquired defects in hemostatic mechanisms.2 Furthermore, with massive bleeding, dilutional hemostatic changes and hypothermia can also occur, and these also contribute to coagulopathy.
Managing bleeding after cardiac surgery requires many preventive and therapeutic considerations.3,4 In high-risk patients, intraoperative measures to prevent bleeding can be used to prevent the adverse effects of CPB and mediastinal suctioning that can produce hemostatic abnormalities.1 Furthermore, tissue injury and increases in stress response can activate fibrinolysis, which produces hemostatic disorders. Pharmacologic therapies to reduce bleeding and the need for allogeneic transfusions have been extensively studied in cardiac surgery and will be reviewed here. These therapies are based on reversing the defects associated with coagulopathy.
Preoperative interventions to reduce blood transfusion begin with identification of high-risk patients who should receive preoperative and perioperative blood conservation measures and for whom antithrombotic drugs should be limited (Box 51-1). Perioperative blood conservation interventions include use of antifibrinolytic drugs, selective use of off-pump coronary artery bypass graft surgery, routine use of a cell-saving device, and implementation of appropriate transfusion modalities. An important intervention is a multimodality blood conservation program that is institution based, that is accepted by all health-care providers, and that involves well-thought-out algorithms to guide transfusion decisions. In this chapter, we focus on coagulation changes that occur in cardiac surgery, therapies to prevent and treat bleeding when it occurs, and blood conservation strategies. Recent concepts in understanding pharmacologic agents are reviewed.
Box 51–1 Predictors of Postoperative Bleeding in Cardiothoracic Surgery
From Ferraris VA, Ferraris SP, Saha SP, et al. Ann Thorac Surg 2007;83:S27-86.
NORMAL HEMOSTATIC MECHANISMS
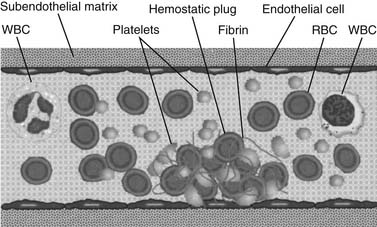
The vascular endothelium, which plays a major role in preventing clotting (Fig. 51-1), is a nonthrombogenic surface that secretes various substances to prevent coagulation from occurring. Prostacyclin (PGI2), tissue plasminogen activator (tPA), heparan sulfate, antithrombin III, protein C, and endothelium-derived relaxing factor (EDRF) are expressed or secreted to inhibit platelet activation and fibrin formation, and to provide vascular patency.5 However, if a blood vessel is cut or otherwise damaged, tissue factor and other molecular promoters are released or exposed to provide a thrombotic surface. Exposure of subendothelial vascular basement membrane activates platelets, and expression of tissue factor also activates the thrombin generation and cellular amplification.6 Another important mechanism for the initiation of the coagulation cascade is platelet activation. Receptors on platelets bind to the damaged blood vessel by forming a bridge with von Willebrand’s factor (vWF) to initiate platelet adhesion.7 Once platelets adhere, they undergo surface receptor changes that cause platelets to aggregate. Once platelets aggregate, they expose factors on their surfaces that provide a template for additional initiation of the coagulation cascade and formation of the early hemostatic plug. Platelets play vital roles in maintaining vascular hemostasis. Any abnormality in platelet number or function poses significant risk for postoperative coagulopathy.
INHIBITING HEMOSTASIS: ANTICOAGULATION
Heparin
Heparin is the primary agent used to prevent clotting during cardiovascular surgery. It is isolated from porcine intestine (previously from beef lung), where it is bound to histamine and stored in mast cell granules. When heparin is isolated, the purification results in a heterogeneous mixture of molecules. Heparin is an acidic molecule with side groups, either sulfates or N-acetyl groups, attached to individual sugar groups; these molecular aspects are important for producing its anticoagulant activity.8,9 Heparin acts as an anticoagulant by binding to antithrombin III (AT), enhancing the rate of thrombin-AT complex formation by 1000 to 10,000 times. Other factors in the clotting cascade, including factor Xa, are also inhibited by AT.9 Anticoagulation thus depends on the presence of adequate amounts of circulating AT.
Heparin anticoagulation can be reversed immediately by removing heparin from AT with the highly basic molecule protamine. Heparin also binds to a number of other blood and endothelial proteins.8 Each of these can potentially influence the ability of heparin to act as an anticoagulant, and each may, along with AT levels, affect heparin dose responses in patients. Heparin can also produce platelet dysfunction after acute or constant administration, especially with high-dose administration during cardiac surgery. Severe adverse reactions to heparin include immune reactions (e.g., hypersensitivity) and the perhaps better-recognized heparin-induced thrombocytopenia (see later).
In January 2008, the Food and Drug Administration received reports of clusters of acute hypersensitivity reactions in patients undergoing dialysis. The Centers for Disease Control and Prevention identified heparin as a common feature of the cases, leading to a recall of particular lots. However, after the initial recall, reports of allergic-type reactions in patients in other clinical settings continued. The contaminant was recently identified as an unusual oversulfated form of chondroitin sulfate (OSCS), representing up to nearly 30% by weight in suspect lots of heparin.10 Highly charged molecules like this can activate enzymatic cascades in plasma. Contaminated lots of unfractionated heparin were found to activate the kinin-kallikrein pathway to produce vasodilation via contact activation.10
Protamine Administration for Heparin Reversal
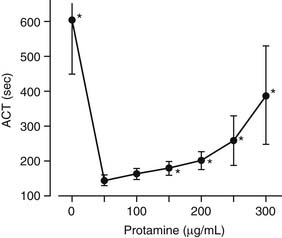
Protamine, the primary heparin-neutralizing agent, is a basic polypeptide isolated from salmon sperm. Composed mostly of arginine, protamine can immediately reverse the anticoagulation effect of unfractionated heparin by a nonspecific acid–base (polyanionic-polycationic) interaction. Different methods can be used to calculate the reversal dose of protamine, but a useful approximation is to use 1.0 to 1.3 mg protamine for each 100 units of unfractionated heparin initially administered.8 Protamine has the potential to function as an anticoagulant, when excessive doses have been administered (Fig. 51-2).11 Additional considerations about protamine and its potential adverse effects will be considered later.
Low-Molecular-Weight Heparin
Low-molecular-weight heparin (LMWH) is a derivative of unfractionated heparin, whose fragments have a mean molecular weight of approximately 5000 daltons. LMWH fragments of less than 18 saccharides retain the critical pentasaccharide sequence needed for formation of the Xa:antithrombin complex.12 It has been suggested that LMWHs provide a therapeutic benefit because factor Xa generation occurs several steps earlier in the coagulation cascade than thrombin generation; inhibition of Xa has a marked effect on the later steps in coagulation. Although the use of LMWH is rapidly increasing in cardiovascular medicine because of its long half-life and ease of dosing, it may pose a problem for cardiac surgical patients because commonly used hemostatic tests are not affected by it. Furthermore, because LMWHs are not readily reversible with protamine, they are not suitable anticoagulants for CPB.
Fondaparinux
Fondaparinux (Arixtra), a synthetic pentasaccharide with a duration of action longer than that of LMWH, selectively binds antithrombin and causes rapid and predictable inhibition of factor Xa.13 Fondaparinux is more effective than enoxaparin in preventing venous thrombosis in patients undergoing orthopedic surgery, and it is similar in effectiveness to enoxaparin or unfractionated heparin in patients with pulmonary embolism.14 Pilot trials involving patients with acute coronary syndromes and patients undergoing percutaneous coronary intervention suggest that fondaparinux may be as effective as enoxaparin, or safer than unfractionated heparin.15 The Fifth Organization to Assess Strategies in Acute Ischemic Syndromes (OASIS-5; NCT00139815) trial compared the efficacy and safety of fondaparinux and enoxaparin (Lovenox, Sanofi-Aventis) in high-risk patients with unstable angina or myocardial infarction without ST-segment elevation.15 The result of this study indicated that fondaparinux reduces the risk of ischemic events as effectively as enoxaparin does, but with lesser risk of major bleeding.
Warfarin
Warfarin, a member of the family of drugs known as vitamin K antagonists, is the most widely available oral anticoagulant. It has major limitations, including slow onset and offset and a narrow therapeutic window, and its metabolism is affected by diet, concomitant drugs, and genetic polymorphisms, and thus it requires careful monitoring.16 Warfarin is a vitamin K analog that interferes with the transformation of coagulation factors to an active form. Vitamin K is required in the post-translational carboxylation required for the synthesis of active coagulation factors II, VII, IX, and X. Without vitamin K, these coagulation factors are incapable of chelating calcium, which is required for their binding to phospholipid membranes during the normal clotting process. The resultant deficient clotting factor activities decrease prothrombin activation. Warfarin also inhibits the carboxylation of protein C and protein S, thus impairing the function of the natural inhibitory anticoagulant proteins. Warfarin is often a mainstay in preventing thromboembolic complication in patients with prosthetic heart valves, atrial fibrillation, atrial mural thrombi, deep vein thrombosis, or prior pulmonary embolic problems. Warfarin is rapidly absorbed from the gastrointestinal tract, with peak plasma concentrations reached 1 to 4 hours after ingestion; the anticoagulant effect of the coumarin becomes visible only after a significant decrease in the concentration of normal vitamin K–dependent clotting factors.8 Because these clotting factors have half-lives of various durations, warfarin therapy is typically initiated in combination with a heparin anticoagulant until the level of the factor with the longest half-life, factor II, has been reduced.
New Oral Anticoagulants
Ximelagatran, the first oral anticoagulant, was withdrawn from the market in Europe because of organ toxicity. Newer oral anticoagulants in advanced stages of clinical development are directed against the active site of factor Xa or thrombin, the enzymes responsible for thrombin generation and fibrin formation, respectively.16 Rivaroxaban and apixaban target factor Xa, whereas dabigatran etexilate inhibits thrombin. Rivaroxaban is a small molecule directed against the active site of factor Xa. After oral administration, it is absorbed in the stomach and small intestine with a bioavailability of 60% to 80%. Peak plasma levels are achieved in 3 hours, and the drug circulates with a half-life of 9 hours.16
Heparin-Induced Thrombocytopenia and New Anticoagulants
Heparin-induced thrombocytopenia (HIT) is an adverse, potentially life-threatening, effect of heparin, produced by antibodies (IgG) to the composite of heparin–platelet factor 4 (PF4) that leads to the formation of immune complexes.17 These immune complexes bind to platelets via platelet Fc-receptors (CD 32), producing intravascular platelet activation, thrombocytopenia, and platelet activation with potential thromboembolic complications that can result in limb loss or death.17 HIT can occur after 5 to 10 days of heparin therapy, but it may occur earlier in cases of occult heparin exposure from prior hospitalizations or in the cardiac catheterization laboratory. HIT develops in 1% to 3% of heparin-treated patients.18
The antibodies that mediate HIT (i.e., heparin–PF4 antibodies) occur more often than the overt disease itself and, even without thrombocytopenia, are themselves associated with increased thrombotic morbidity and mortality.18 HIT should be suspected whenever the platelet count drops more than 50% from baseline after starting heparin (or sooner if there was prior heparin exposure), or when new thrombosis occurs during, or soon after, heparin treatment, with other causes excluded. When HIT is strongly suspected, with or without complicating thrombosis, heparins should be discontinued, and a fast-acting, nonheparin alternative anticoagulant, such as a direct thrombin inhibitor (argatroban or r-hirudin) or danaparoid, should be initiated immediately.18,19
As noted previously, even without inducing thrombocytopenia, heparin-PF4 antibodies may increase morbidity or mortality in various patient populations. In patients with, versus without, heparin-PF4 antibodies, regardless of the platelet count, there are significant increases in the hospitalization and in-hospital mortality after cardiac surgery20 and other surgeries. Despite their association with long-term adverse effects, circulating heparin-PF4 antibodies are transient, although they are very likely recurrent with repeated heparin exposure. The agents currently recommended or approved for typical use in patients with HIT are the direct thrombin inhibitors, specifically argatroban, or danaparoid.19 For cardiac surgery, bivalirudin has emerged as the agent most studied in this setting, for on- or off-pump surgery.21,22 For patients who have HIT and are under intensive care, fondaparinux is a potential alternative for prophylaxis, and eventually the patient should be switched to warfarin when long-term anticoagulation is desired.17
ACQUIRED PLATELET DYSFUNCTION
Antiplatelet agents are the primary therapy for patients with atherosclerotic vascular disease and coronary artery disease; this therapy is consistent with the role of platelets in atherosclerosis.23 Treatment with aspirin reduces the incidence of occlusive arterial vascular events. Aspirin irreversibly acetylates cyclooxygenase and thereby prevents formation of thromboxane A2, a prostaglandin that mediates the activation of more platelets. Clopidogrel is a thienopyridine that inhibits the P2Y12 receptor, and it is widely used in patients with atherosclerotic vascular disease and in patients who do not tolerate aspirin.23 The combination of aspirin plus clopidogrel is recommended after coronary stenting and for up to 9 months in patients with acute coronary syndrome.23 These drugs and other anticoagulant therapies are associated with excessive intraoperative and postoperative bleeding, as well as resultant transfusions, in most but not all situations.24–26 Patients with thrombocytopenia or with qualitative platelet defects (e.g., renal failure, von Willebrand’s disease) may be at greater risk for bleeding. Discontinuation of antiplatelet and antithrombotic drugs before cardiac surgery in these high-risk patients should be considered.
Clopidogrel and aspirin therapy result in a higher rate of postoperative bleeding, the use of more transfused blood products, and a higher rate of reexploration for mediastinal hemorrhage during emergency coronary artery bypass grafting surgery (CABG).27–29 Joint guidelines from the American College of Cardiology and the American Heart Association (ACC/AHA) and the current guidelines from the Society of Thoracic Surgeons (STS) recommend stopping adenosine diphosphate (ADP) inhibitors 5 to 7 days before cardiac operations, if possible, recognizing that operations sooner than 5 days in patients on ADP inhibitors risk increased perioperative bleeding and transfusions and possibly worse long-term outcomes.30 However, in patients with drug-eluting stents, the abrupt discontinuation of platelet inhibitors may also increase the risk for thrombotic events, and there is little evidence to guide therapy in this situation; discussion with all members of the cardiovascular team—cardiologists, cardiac surgeons, anesthesiologists, and the operating team—is warranted. New shorter-acting antiplatelet agents under investigation (e.g., cangrelor) may offer an important therapy, but for now, changing to a glycoprotein (GP) IIb/IIIa inhibitor or to a direct thrombin inhibitor may be an alternative.24
Additional therapeutic agents may produce platelet dysfunction. Platelet glycoprotein (GP) IIb/IIIa complexes are important in platelet-mediated thrombus formation and have been used as therapeutic strategies to treat acute coronary thromboses, probably less commonly because of the increased use of clopidogrel.31 Three different GP IIb/IIIa antagonists are available, and they differ in antagonist affinity, reversibility, and receptor specificity. GP IIb/IIIa (IIbβ3) is a receptor on platelets that binds to key hemostatic proteins, including fibrinogen and vWF, to allow cross-linking of platelets and platelet aggregation. By blocking this final common pathway using GP IIb/IIIa antagonists, these drugs function as inhibitors of platelet participation in acute thrombosis.
HEMOSTATIC TESTING
Hemostatic testing is often used preoperatively to identify patients at risk for bleeding and to better define the specific defect producing bleeding. Although platelet dysfunction is a major cause of bleeding after cardiac surgery, appropriate laboratory evaluation of platelet dysfunction is not widely available. Hemostatic testing would help identify the best pharmacologic and transfusion-based therapy, but most platelet function tests available for point-of-care testing or in the laboratory have not been suitably validated for cardiac surgical patients, and non-point-of-care testing is not readily available. Furthermore, dilutional thrombocytopenia may affect the test results. Better tests of platelet dysfunction are needed to more accurately diagnose the underlying disorder in this patient population.32
Despite the lack of studies supporting platelet function tests in the perioperative management of cardiac surgical patients, many studies have shown that using algorithms based on point-of-care coagulation tests can decrease bleeding and transfusion requirements after cardiac surgery. An important caution is that hemostatic tests may be abnormal in patients who are not bleeding. Transfusion algorithms can be used to guide intraoperative transfusion and may prevent or decrease the empiric administration of hemostatic factors.33 Cardiac surgery services should use transfusion guidelines based on laboratory-guided algorithms, and the possible benefits of point-of-care testing should be tested against this standard.
Risk Factors for Bleeding
Although most studies do not distinguish between red blood cell transfusion and hemostatic factor transfusion, Ferraris and colleagues24 summarized the variables associated with increased transfusion requirements caused by patient-related, procedure-related, and process-related factors in their inclusive review. They identified a high-risk profile associated with increased postoperative blood transfusion, and six variables stand out as important indicators of bleeding risk: (1) advanced age, (2) low preoperative red blood cell volume (related to preoperative anemia or small body size), (3) preoperative antiplatelet or antithrombotic drugs, (4) reoperative or complex procedures, (5) emergency operations, and (6) noncardiac patient comorbidities. Risk factors are summarized at the Annals of Thoracic Surgery website, available at http://ats.ctsnetjournals.org/cgi/content/full/83/5_Supplement/S27.
Patient-Related Causes of Bleeding
Patients at greater risk for bleeding include those with acquired or congenital coagulopathies, those scheduled for complex procedures (e.g., combined valve and coronary revascularization, aortic dissection with deep hypothermic circulatory arrest), those having repeat cardiac procedures, those with sepsis with thrombocytopenia, and those who are Jehovah’s Witnesses. There is evidence that certain patients have an accentuated response to antiplatelet drugs.34 Patients with thrombocytopenia from whatever cause (defined as a platelet count below 50,000) are at high risk for excessive bleeding after CABG, but this depends on the variety of platelet dysfunction and is difficult to measure. Patients with preoperative anemia have a lower starting red blood cell mass going into surgery, and anemia can contribute in complex ways to bleeding. Patients with other congenital or acquired qualitative platelet defects, such as von Willebrand’s disease, Bernard-Soulier syndrome, and Glanzmann’s thrombasthenia, are at increased risk for bleeding.24 Acquired qualitative defects occur with hepatic and renal failure and are commonly drug induced.
Physician-Related Causes of Bleeding
Another factor in surgical bleeding, and thus blood transfusion, is the surgeon. Surgical practices differ widely and influence morbidity and mortality.35 Differences in CPB practices, such as time on bypass, can affect platelet function and perioperative bleeding. Surgeons define therapy differently, and even their different degrees of exploration for excessive postoperative hemorrhage contribute to variability in transfusion practices.36 Furthermore, transfusion practices vary among centers.24,37,38
Procedure-Related Causes of Bleeding
Certain procedure-related factors increase risk for bleeding and perioperative morbidity and mortality. Repeat procedures have higher transfusion rates, and the type and urgency of the surgery are independent predictors for transfusion.39–42 CPB also influences platelet function and coagulation.42 Although off-pump cardiac surgery is associated with an overall reduction in transfusion, these patients, because of the extensive surgery, blood loss, and hemodilution, can also need transfusion.43,44 Complex, long procedures (e.g., bilateral internal mammary artery grafts, aortic valve replacement with a pulmonary autograft [Ross procedure], surgery related to ventricular-assist devices or artificial hearts) are associated with greater risk for bleeding.24
Drug-Related Causes of Bleeding
Therapy for the prevention or treatment of cardiovascular disease involves maximizing platelet function, inhibiting clot formation, and causing clots to lyse. When patients who need surgery are on these agents, there is a greater risk for bleeding.24 However, this may not be the case for patients who are on warfarin and undergoing CPB, because of better thrombin inhibition.45 Ferraris and coworkers note that preoperative antiplatelet and anticoagulant treatment as prophylaxis for coronary occlusive disease is associated with excessive intraoperative and postoperative bleeding (with resultant transfusion) in most but not all situations, so this aspect of preoperative medication regimens must be managed for maximal cardioprotective benefit while minimizing risk for hemorrhagic complications.24 As mentioned, clopidogrel and aspirin therapy result in higher postoperative bleeding, use of more transfused blood products, and a higher rate of reexploration for mediastinal hemorrhage during emergency CABG27–29 (see Acquired Platelet Dysfunction, earlier, for ACC/AHA and STS guidelines).
TRANSFUSION THERAPY AND TRANSFUSION GUIDELINES
The appropriate use of either red blood cell transfusions or platelet components for cardiac surgical patients continues to be defined. Physicians make transfusion decisions on the basis of limited objective data, using clinical judgment and what they learned in their training. Guidelines and transfusion algorithms for the management of bleeding in cardiac surgical patients have been reported.40,46 Bleeding and reexploration in cardiac surgery are consistently associated with adverse outcomes.47–49 As a result, patients with bleeding are often transfused. However, the lack of consistent evidence-based medicine supporting the decision to transfuse is illustrated by the wide ranges in the rates of blood product use in patients undergoing CABG: from 3% to 83% for red cell products, and from 0% to 40% for platelets.37,38
Unfortunately, most physicians have difficulty following the guidelines and often resort to empiric therapy, often because laboratory tests take too much time, are difficult to obtain, or cannot identify platelet defects, or because of concern about later getting blood products from the blood bank in a timely fashion. Transfusion algorithms have been developed using institution-derived transfusion practices with point-of-care testing to guide therapeutic approaches to bleeding and transfusion. In randomized studies, the use of point-of-care testing and transfusion algorithms decreases the rate of transfusions and improves hemostasis.50–53 Although different point-of-care tests were used in the studies, it is unclear whether the algorithms developed for guiding transfusion and the multidisciplinary approach are more important than the point-of-care testing used because empiric transfusions are eliminated. Ferraris and colleagues note that a multimodality approach is more important than the individual components of the process.24,25
The American Society of Anesthesiologists (ASA) established the Task Force on Blood Component Therapy to develop evidence-based indications for transfusing red blood cells, platelets, fresh-frozen plasma, and cryoprecipitate in perioperative settings.54 Guidelines were developed according to an exact methodology. The recommendations of the task force (see www.asahq.org/publicationsAndServices/transfusion.pdf) are listed in Box 51-2. These guidelines are recommendations, but there are concerns about specific blood components for cardiac surgical patients, as discussed in the following paragraphs.
Box 51–2 Guidelines for Transfusion of Red Blood Cells, Platelets, Fresh-Frozen Plasma, and Cryoprecipitate
Modified from Practice Guidelines for Blood Component Therapy. Anesthesiology 1994;84:732-47.
Red Blood Cells
Unfortunately, there is no red cell transfusion standard based on a single minimum acceptable hemoglobin for all patients. Chronic anemia is better tolerated than acute anemia, but with acute anemia, compensatory mechanisms that increase cardiac output and improve oxygen transport depend on the patient’s cardiovascular reserve, which may be diminished in cardiac surgical patients with heart failure or flow-restricting lesions. Factors to be considered before transfusing red blood cells (RBCs) include intravascular volume status, ongoing rate and amount of active bleeding, and the need for augmented oxygen transport (e.g., after cardiac surgery requiring multiple inotropes and an intra-aortic balloon pump, patients who are anemic may need RBCs). Thus, the need for transfusion must balance the risks against the need for oxygen-carrying capacity in recovery from trauma, surgery, or illness, as noted in the ASA guidelines for perioperative blood transfusion and adjuvant therapies.55 The task force noted in its recommendations that a transfusion of “red blood cells should usually be administered when the hemoglobin concentration is low (e.g., less than 6 g/dL in a young, healthy patient), especially when the anemia is acute. Red blood cells are usually unnecessary when the hemoglobin concentration is more than 10 g/dL. These conclusions may be altered in the presence of anticipated blood loss” or active critical or target organ (i.e., myocardium, central nervous system, or renal) ischemia. “Determining whether intermediate hemoglobin concentrations (i.e., 6-10 g/dL) justify or require RBC transfusion should be based on any ongoing indication of organ ischemia, potential or actual ongoing bleeding (rate and magnitude), the patient’s intravascular volume status, and the patient’s risk factors for complications of inadequate oxygenation. These risk factors include a low cardiopulmonary reserve and high oxygen consumption.”55 Hemoglobin triggers for transfusion are not to be taken as absolute indications, and cardiac patients should be transfused if signs or symptoms of inadequate myocardial oxygenation are present. One important aspect of adverse events associated with RBC transfusions may relate to the age of the RBCs transfused, although large prospective randomized clinical trials remain to be conducted.56–58
Fresh-Frozen Plasma
After RBCs and platelets are removed from a unit of blood, plasma remains. That 170 to 250 mL, containing blood coagulation factors, fibrinogen, and other plasma proteins, is then frozen and can be stored for up to 1 year. Before being administered, the fresh-frozen plasma (FFP) must be thawed in a waterbath at 37° C, which takes about 30 minutes. After thawing, a unit of FFP is stored at 1° to 6° C and is transfused within 24 hours. FFP should be administered through a component administration set with a 170-micron filter. If not used within 24 hours, it can be relabeled as thawed plasma and stored at 1° to 6° C for an additional 4 days. Thawed plasma maintains normal levels of all factors except factor V, which falls to 80% of normal, and factor VIII (FVIII), which falls to 60% of normal.59 Because these levels are above the in vivo threshold for normal hemostatic function, and FVIII is an acute-phase reactant, thawed plasma can be used as a substitute for FFP.59
FFP is traditionally used for treating bleeding resulting from coagulopathies that involve a prolongation of either activated partial thromboplastin time (aPTT) or prothrombin time and International Normalized Ratio (PT/INR) greater than 1.5 times normal, or a coagulation factor assay of less than 25%.55 FFP is often used to reverse the effect of warfarin before surgery or during active bleeding episodes (see Reversal of Vitamin K Antagonist–Associated Coagulopathy, later). When FFP is indicated, it should be administered in a dosage calculated to achieve a minimum of 30% of plasma factor concentration. A dosage of 10 to 15 mL/kg of FFP generally results in a rise of most coagulation proteins by 25% to 30% (or increases in 0.25 to 0.3 U/mL), although a dosage of 5 to 8 mL/kg may be adequate if needed to urgently reverse warfarin anticoagulation, but this varies with the initial levels of the vitamin K–dependent coagulation factors.55 FFP is also part of a transfusion algorithm for post-traumatic bleeding.
Cryoprecipitate
In Europe, specific fibrinogen concentrates are available for fibrinogen replacement therapy. However, one unit of cryoprecipitate per 10 kg of body weight increases plasma fibrinogen by roughly 50 to 70 mg/dL without contributing to consumption or massive bleeding.60,61 The minimum hemostatic level of fibrinogen is traditionally suggested to be around 100 mg/dL, but normal fibrinogen levels are 200 mg/dL and higher, and these higher levels of fibrinogen may be important for clot formation (see Fibrinogen, later). Because cryoprecipitate does not contain factor V, it should not be the sole replacement therapy for patients with disseminated intravascular coagulopathy, which is almost always associated with various factor deficiencies and thrombocytopenia, as noted in the guidelines from the American Association of Blood Banking (AABB) (Available at www.AABB.org). Because fibrinogen is an important determinant of hemostatic function and clot strength, fibrinogen levels should be routinely evaluated in bleeding patients, especially after multiple transfusions. Hypofibrinogenemia itself can cause a prolonged PT and PTT, and FFP transfusion alone may not provide sufficient repletion. Cryoprecipitate is probably underutilized in cardiac surgical patients who are bleeding and refractory to standard FFP and platelets.
Massive Transfusion
Massive transfusion is defined as the acute replacement of more than one blood volume, or the use of more than 10 units of packed RBCs within several hours. In the acute clinical setting, the transfusion of four or more red cell units within 1 hour when ongoing need is foreseeable, or replacing 50% of the total blood volume within 3 hours, may be more appropriate.61,62 The most common clinical situation leading to massive transfusion is extensive trauma; however, massive transfusion may also occur in nontrauma settings during surgical procedures causing large blood loss, especially after cardiothoracic surgery.61,62 Extensive information has been learned from the Iraq war in this area.63–65 Blood transfusion is a main therapy option for treating acute hemorrhage. However, for trauma patients, ideal repletion is with fresh whole blood, which is not widely available. The etiology of coagulopathy during massive transfusion is complex and involves dilutional factors, hypothermia, tissue hypoperfusion/ischemia, acidosis, and potential disseminated intravascular coagulation; this syndrome may occur in cardiac surgical patients, too. Treatment of the coagulopathy should include volume replacement, normothermia, resolution of acid–base abnormalities and blood component therapy, and correction of hypocalcemia. Because of fibrinolysis, an antifibrinolytic should be considered for the cardiac surgical patient. The role of off-label use of recombinant activated factor VII (see Recombinant Factor VIIa, later) to manage bleeding that cannot be controlled by conventional measures is still evolving.
ADVERSE EFFECTS OF TRANSFUSIONS
The risks of allogeneic transfusion extend beyond viral transmission and include allergy, alloimmunization, bacterial sepsis, graft-versus-host disease, transfusion-related acute lung injury (TRALI), renal failure, volume overload, and immunosuppression.66–68 Beside possible bacterial contamination, platelet concentrates for transfusions contain a high concentration of donor white blood cells, which can produce multiple adverse effects. Cytokines, such as interleukins 6 (IL-6) and 8 (IL-8), tissue necrosis factor alpha (TNFα), and other inflammatory mediators especially concentrated in platelet products could contribute to adverse outcomes.
Platelet concentrates without leukodepletion have been found to be a proinflammatory mixture.69 Although similar work has not been performed with platelet transfusion, the levels of cytokines, TNFα, IL-6, and IL-8 are increased 100- to 1000-fold over baseline in platelet products.69 As complement and cytokines depress platelet function and increase the release of tPA, the large influx of proinflammatory mediators from platelet transfusion might mitigate some of the procoagulant function of platelets, increase bleeding, or make platelet transfusions ineffective.70
Even though active white cells are responsible for forming the high levels of complement and cytokine factors, leukoreduction may be only partially effective in reducing the immunosuppressive effects of platelets.71 In red cell transfusion, leukoreduction may affect T-cell activation and expression of key immune molecules on the surface of white cells.72 Other mechanisms of immunosuppression not affected by leukoreduction may come into play. The presence of free, nonprotein-bound iron leads to the activation of white cells (whether allogeneic or autologous).73 Furthermore, leukoreduced platelet units that are stored at room temperature today may increase the number of septic platelet transfusion units. The effect of bacterial cell wall material on the systemic proinflammatory processes seen during CPB is not clear.
Platelet transfusions have long been known to possess a storage lesion leading to decreased effectiveness compared with native circulating platelets.74 Large percentages of platelets may already be hemostatically “dead” and functioning only as cellular shells. This lack of normal cellular activity and regulation may play some role in increasing adverse events. For example, infusion of a platelet product into a patient with an embolic nidus could potentiate thrombus or cerebral infarction. Similarly, a prothrombotic potential in a reperfused coronary or cerebral artery could promote thrombus generation if platelet transfusion were additive to thrombotic tendencies. This is best reflected by a study demonstrating that platelet transfusions were associated with infection, vasopressor use, respiratory medication use, stroke, and death.69
Transfusion-Related Acute Lung Injury
TRALI is one of the most life-threatening adverse effects of transfusion. Although the true incidence of trali is unknown, it is the leading cause of transfusion-related death according to the United States Food and Drug Administration (FDA).75 Clinical presentation of TRALI, in its severe form, is indistinguishable from adult respiratory distress syndrome (ARDS) and is characterized by acute onset (within minutes, to 1 to 2 hours after transfusion), bilateral pulmonary infiltrates, and hypoxia without evidence of heart failure.66,76,77 TRALI may resolve faster and has a lower mortality rate than ARDS. TRALI usually develops within 6 hours (most often less than 2 hours) of a transfusion, usually resolves within 24 to 48 hours, and has a mortality rate of around 5% to 10%, whereas ARDS does not usually develop until at least 24 hours after exposure to a risk factor, has a duration often longer than 72 hours, and has a mortality approaching 30% to 60%.66 Because of increasing awareness and identification of TRALI, and also because of decreases in the incidence of infectious and hemolytic complications of transfusions, TRALI is now a primary cause of transfusion-associated mortality reported to the FDA and has become a frequent cause of transfusion-related morbidity.78 It can occur after the transfusion of RBCs, but it is most often seen after transfusion of the plasma-containing blood components such as FFP and platelets. TRALI can be confused with other transfusion- and non-transfusion-related events such as anaphylaxis, hemolysis, circulatory overload, and cardiac failure, as patients present with acute shock, florid pulmonary edema, and pulmonary hypertension.79,80 Risk estimates suggest it occurs once in 8000 to 70,000 transfused units.66
Depending on the causative factor in the transfused component and the inflammatory state of the pulmonary circulation after cardiac surgery, two different (but at times complementary and overlapping) pathogenic mechanisms are thought to cause TRALI: the classic antibody-mediated for most, and the two-hit inflammatory insult for some.66,79,81 Most cases of TRALI are caused by passive transfer of donor-related antileukocytic antibodies directed at human leukocyte antigen (HLA) or granulocyte-specific antigens on the patient’s leukocytes.66 This promotes priming and activation of a patient’s granulocytes, leading to their pulmonary sequestration and release of proteases, oxidants, and leukotrienes, which cause alveolar epithelial and microvascular endothelial damage, resulting in increased permeability and an eventual development of noncardiogenic pulmonary edema. The two-hit model of TRALI is similar to that which has been proposed to cause ARDS. With TRALI, however, the specific causative agent in the blood component is unknown, although there is growing evidence associating bioactive factors or white cell priming lipids, CD40 ligand released by platelets, or several reactive lipid-like substances accumulating in red blood cells or platelets during storage. These compounds, called biological response modifiers, can be the first pulmonary insult but are more likely the second. The first insult or hit is generally a systemic inflammatory condition secondary to major surgery, sepsis, trauma or pulmonary aspiration that causes activation of the pulmonary endothelium and polymorphonuclear lymphocytes (PMN) priming, leading to their sequestration in the pulmonary vasculature. The second hit occurs when the primed PMNs are activated by the biological response modifiers in the transfused component.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree