The aim of the present study was to investigate the long-term effects of alcohol septal ablation (ASA) on left ventricular (LV) and right ventricular (RV) remodeling in patients with obstructive hypertrophic cardiomyopathy (HC) using cardiovascular magnetic resonance (CMR). CMR was performed at baseline and 16 months after ASA in 38 patients with obstructive HC (mean age 48 ± 9 years) despite optimal medical treatment. ASA resulted in significant reductions of LV outflow tract gradient (mean 89 ± 22 vs 24 ± 12 mm Hg, p <0.001) and improvements in New York Heart Association functional class (p <0.001) during the follow-up period. LV remote mass and septal mass decreased from 98.34 ± 37.02 to 84.23 ± 34.71 g and from 77.56 ± 16.40 to 68.43 ± 14.02 g, respectively (p <0.001 for both) at 16-month follow-up. There were significant reductions of RV mass (mean 53.69 ± 7.12 vs 47.49 ± 6.17 g, p <0.001) and improvements in RV end-diastolic volume (mean 110.58 ± 22.47 vs 124.22 ± 24.17 ml, p <0.001) and the RV ejection fraction (p <0.001) during 16-month follow-up. Linear regression analysis showed that LV outflow tract gradient reduction was correlated significantly with LV remote mass reduction (r = 0.475, p = 0.003) and RV mass reduction (r = 0.535, p = 0.001) at 16-month follow-up. In conclusion, successful ASA can lead to positive biventricular reverse remodeling, showing significant reductions of RV and LV mass as well as increased RV and LV end-diastolic volumes during follow-up.
Hypertrophic cardiomyopathy (HC) is the most common genetic disease associated with left ventricular (LV) hypertrophy, which commonly involves the LV septum and dynamic LV outflow tract (LVOT) obstruction. Alcohol septal ablation (ASA) is an established alternative technique to surgical myectomy for the reduction of LVOT gradient and subsequent alleviation of refractory symptoms in patients with obstructive HC. Previous studies have demonstrated regressions of septal thickness and remote LV mass after successful reduction of the LVOT gradient. Although most clinical evaluations focus on LV remodeling after ASA, only limited data are available on biventricular remodeling changes after successful ASA. Cardiovascular magnetic resonance (CMR) imaging can provide accurate images of both ventricles because of high spatial and temporal resolution and is ideally suited for evaluation of right ventricular (RV) structure and function. Therefore, the aim of the study was to assess biventricular remodeling and function during 16-month follow-up after successful ASA.
Methods
The study protocol was approved by the Institutional Ethics Committee of Fuwai Hospital. All patients gave informed consent before enrollment in the study. We studied 38 patients with obstructive HC who underwent ASA procedures from August 2005 to December 2012. Indications for ASA included severe, drug-refractory, New York Heart Association functional class III or IV heart failure with an LVOT gradient >50 mm Hg at rest or >100 mm Hg during provocation. No patients had previous surgical myectomy, aortic valvular disease, intrinsic mitral disease, or general contraindications to CMR.
The ASA procedure was performed using a method previously described, under continuous electrocardiographic and blood pressure monitoring. In brief, through a 6Fr or 7Fr left coronary guiding catheter, an oversized over-the-wire angioplasty balloon (diameter 1.5 to 2.5 mm) was placed in the proximal part of the septal perforator artery. After the balloon was inflated (pressure 6 bar), the supply area of the closed septal branch was determined by injection of angiographic contrast together with simultaneous transthoracic echocardiographic registration. After delineation of the size of targeted myocardium, 1 to 3 ml of ethanol was slowly injected. A successful procedure was defined by a reduction in the LVOT pressure gradient of >50% of the baseline value.
CMR imaging studies were performed with a 1.5-T speed clinical scanner. All images were acquired with an electrocardiographically gated breath-hold technique. First, steady-state free precession cine images were acquired in 3 long-axis 4-chamber, 3-chamber, and 2-chamber long- and short-axis views with the following protocol: repetition time 2.7 ms, echo time 1.2 ms, flip angle 70°, temporal resolution 40 ms, field of view 360 × 315 mm 2 , matrix size 192 × 162; slice thickness 6 mm, and slice gap 2 mm. Finally, late gadolinium enhancement images were acquired 10 minutes after the intravenous administration of 0.2 mmol/kg gadodiamide hydrate using a segmented phase-sensitive inversion-recovery spoiled gradient-echo sequence.
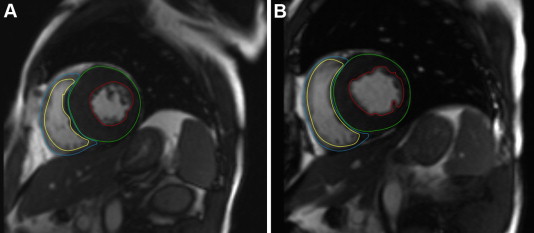
All CMR image analysis was performed by experienced radiologic technicians using dedicated software (Medis Medical Imaging Systems, Leiden, the Netherlands). Epicardial and endocardial borders of the LV and RV myocardium before and after ASA were manually traced during the whole cardiac phase on each cine short-axis image to obtain LV and RV end-diastolic and end-systolic volumes, ejection fractions, and myocardial mass ( Figure 1 ). Myocardial mass was calculated by multiplying the volume of the myocardium calculated at end-diastole by the specific gravity of the myocardium (1.05 g/ml). The end-diastolic volume index, end-systolic volume index, and mass index were indexed to body surface area. Infarct size after ASA was evaluated by manual tracing of the hyperenhanced area, which was defined as the area within the septal myocardium with pixel signal intensity >4 SDs of remote, nonenhanced myocardium. Central dark zones within the area of hyperenhancement were included. Interventricular septum was defined as the myocardium between the anterior and posterior junctions of the right ventricle to the left ventricle. Remote LV mass was measured as LV mass minus interventricular septal mass. Mitral regurgitation severity was measured by color Doppler and grading by vena contracta width and proximal isovelocity surface area according as follows: 1+ = mild, 2+ = moderate, 3+ = moderate to severe, and 4+ = severe.
Data are expressed as mean ± SD for normally distributed continuous variables and as medians and interquartile ranges for non–normally distributed continuous variables. Differences between means were measured by paired Student’s t tests. Wilcoxon-Mann-Whitney U tests were used to examine differences in medians. Noncontinuous data were compared by chi-square or Fisher’s exact tests as appropriate. The relations between the changes of parameters were assessed using linear regression analysis. Statistical significance was defined as p <0.05. Statistical analysis was conducted using SPSS version 18.0 for Windows (SPSS, Inc., Chicago, Illinois).
Results
The study cohort had a mean age of 48 ± 9 years, and 28 of the 38 patients (74%) were men. Thirteen patients (34%) had angina pectoris, 8 (21%) had syncope, 29 (76%) had New York Heart Association functional class III or IV symptoms, and 8 (21%) had family histories of HC or sudden cardiac death ( Table 1 ). The median follow-up time was 16 months (range 9 to 39).
| Variable | (n=38) |
|---|---|
| Age (years) | 48 ± 9 |
| Male | 28 (74%) |
| Angina pectoris | 13 (34%) |
| Dyspnea | 34 (89%) |
| Syncope | 8 (21%) |
| NYHA functional class III/ IV | 28 (73%) |
| Family history of HC or sudden death | 8 (21%) |
| Hypertension | 9 (24%) |
| Diabetes | 2 (5%) |
| Atrial fibrillation | 4 (11%) |
| Medications | |
| β-blockers | 32 (84%) |
| Calcium channel antagonist | 10 (26%) |
| ARB/Antiarrhythmic drugs/diuretics | 5 (13%) |
ASA resulted in significant reductions of LVOT gradient at rest in all patients. The mean LVOT gradient decreased from 89 ± 22 to 34 ± 23 mm Hg (p <0.001) after the procedure and further decreased from 34 ± 23 to 24 ± 12 mm Hg (p <0.001) during the 16-month follow-up period ( Figure 2 ). The favorable LVOT gradient reduction was associated with significant improvement of functional status: 35 patients (92%) had improved New York Heart Association functional class at follow-up ( Figure 3 ). The median New York Heart Association functional class improved from 3 (1 to 4) to 1 (1 to 3) (p <0.001) at follow-up. Furthermore, mitral regurgitation severity reduced significantly in 33 patients (87%) ( Figure 3 ). The median mitral regurgitation severity grade decreased from 2+ (0 to 4) at baseline to 1+ (0 to 2) at follow-up (p <0.001).
At 16-month follow-up, a clearly demarcated area of hyperenhancement was seen in the basal part of the interventricular wall in all patients. The mean myocardial infarct size was 13 ± 5 g and involved 19% of the septal mass at 16 months after ASA. A larger infarct size was associated with a larger decrease of septal mass (r = −0.467, p = 0.003) as well as LVOT gradient reduction (r = −0.632, p <0.001) at follow-up. Septal thickness reduced from 20 ± 1 mm at baseline to 15 ± 1 mm (−5 mm, p <0.001) at follow-up. LV end-diastolic dimension increased from 46 ± 3 to 48 ± 3 mm (p <0.001) at follow-up. LV mass, septal mass, and LV remote mass all decreased significantly (−13%, −12%, and −14%, respectively (p <0.001 for all)) at follow-up. Furthermore, LV end-diastolic volume and LV end-diastolic volume index increased significantly (p <0.001 for both) at follow-up. There was no significant difference (p = 0.324) in the LV ejection fraction between baseline and follow-up ( Table 2 ).
| Baseline (n = 38 ) | Follow-up (n = 38 ) | p-Value | |
|---|---|---|---|
| Septal thickness (mm) | 20 ± 1 | 15 ± 1 | <0.001 |
| Posterior wall (mm) | 10 ± 2 | 9 ± 1 | <0.001 |
| Left ventricular end-diastolic diameter (mm) | 46 ± 3 | 48 ± 3 | <0.001 |
| Total left ventricular mass (g) | 176 ± 51 | 153 ± 46 | <0.001 |
| Septal mass (g) | 78 ± 16 | 68 ± 14 | <0.001 |
| Remote mass (g) | 98 ± 37 | 84 ± 34 | <0.001 |
| Left ventricular mass (g/m 2 ) | 96 ± 24 | 85 ± 22 | <0.001 |
| Left ventricular end-diastolic volume (ml) | 135 ± 26 | 147 ± 28 | <0.001 |
| Left ventricular end-diastolic volume index (ml/m 2 ) | 74 ± 12 | 80 ± 12 | <0.001 |
| Left ventricular stroke volume (ml) | 96 ± 21 | 103 ± 21 | <0.001 |
| Left ventricular stroke volume index (ml/m 2 ) | 52 ± 10 | 56 ± 10 | <0.001 |
| Left ventricular ejection fraction (%) | 71 ± 5 | 70 ± 4 | 0.324 |
RV mass and RV mass index decreased significantly (−7 g and −2 g/m 2 , p <0.001 for both). In contrast, RV end-diastolic volume and RV end-diastolic volume index increased significantly (by 13 ml and 7 ml/m 2 , respectively, p <0.001 for both). The RV ejection fraction increased significantly from 40 ± 3% to 46 ± 3% (p <0.001) during follow-up ( Table 3 ).
| Baseline (n = 38 ) | Follow-up (n = 38 ) | p-Value | |
|---|---|---|---|
| Right ventricular mass (g) | 54 ± 7 | 47 ± 6 | <0.001 |
| Right ventricular mass index (g/m 2 ) | 29 ± 4 | 27 ± 4 | <0.001 |
| Right ventricular end-diastolic volume (ml) | 111 ± 22 | 124 ± 24 | <0.001 |
| Right ventricular end-diastolic volume index (ml/m 2 ) | 60 ± 10 | 67 ± 11 | <0.001 |
| Right ventricular stroke volume (ml) | 44 ± 11 | 57 ±12 | <0.001 |
| Right ventricular stroke volume index (ml/m 2 ) | 24 ± 5 | 31 ± 6 | <0.001 |
| Right ventricular ejection fraction (%) | 40 ± 3 | 46 ± 3 | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


