Bifurcation Lesions and Intervention
Michael S. Levy MD, MPH
Issam D. Moussa MD
The topic of bifurcation coronary intervention has been one of great controversy since the inception of coronary an-gioplasty in the late 1970s. Meier and Gruentzig published on this topic early on and were dismayed at the rate of side branch occlusion and dissection that resulted in this group of coronary lesions (1). In clinical practice, bifurcation lesions are felt to represent approximately 20% of coronary interventions (2). However, prior to the stent era there was concern in general since there were no modalities that adequately dealt with dissection or abrupt closure of either a main branch or a side branch vessel. In addition, studies had shown that this lesion subset met with an overall lower rate of success and a higher overall rate of restenosis compared with lesions that did not involve bifurcations (3). As the era of in-terventional cardiology has changed, and with the advent of stents, operators were given new tools with which to tackle this problem. Early evidence showed that stenting would improve outcomes in this group of patients (4).
Initial improvement over angioplasty alone was seen with the advent of bare metal stents, but studies still demonstrated a high rate of restenosis (especially at the side branch ostium) and side branch occlusion with these particular stents (4, 5). Studies from this era cite side branch occlusion rates as high as 19% (6) and rates of side branch stenoses of 27% (7) once a main branch stent was initially deployed. Drug eluting stents (DES) appeared to be successful tools to deal with restenosis, and further studies demonstrated the benefit to DES in dealing with restenosis, and in turn target lesion revascularization (TLR) and target vessel revas-cularization (TVR) in this subset of coronary lesions (8, 9 and 10). Early studies with DES suggested that DES were preferable to bare metal stents (BMS) in this lesion subset (11) and that sirolimus conferred benefit over paclitaxel stents.
However, controversy still remains within this topic. A number of trials dedicated specifically to bifurcation stenting have been completed over the last several years, providing further evidence within this field to help inform clinical decision making. However, as multiple classification schemes, multiple techniques, and even new stent technology (including stents specifically designed for bifurcation lesions) are created, it is becoming more and more apparent that this subset of lesions is a heterogeneous group. Not all bifurcations are created equal, and it is therefore difficult to provide a simple “cook book” approach to these lesions. Because of this, it is necessary to review the literature on this topic, including the classification schemes, the seminal trials, and the advice from the current guidelines, in order to provide a balanced treatment of this topic, as well as some potential approaches for dealing with this patient population in clinical practice.
PATHOPHYSIOLOGIC CONSIDERATIONS IN BIFURCATION LESIONS
Ongoing research has sought to examine what determines plaque distribution within coronary artery bifurcations. Studies suggest that much has to do with exposure to shear stress. Areas of low shear stress, which is nonuniform, can lead to plaque accumulation as compared with areas of high, uniform shear stress (12). This theory helps to explain why we see lesion formation around bifurcations in the first place.
With the advent of angioplasty and stent technology, further tools were created to help tackle these complex lesions. However, the challenge is that these lesions are more prone to restenosis than nonbifurcation lesions. Interestingly, two different mechanisms are proposed: When stenting has been performed, the most common reason for lumen loss over time is neointimal formation. This is felt to account for 90% of the restenotic process. Neointimal formation is felt to account for less than 30% of the restenotic mechanism in balloon-only angioplasty (13). In contrast, the major mechanism of restenosis for balloon angioplasty is from elastic recoil and late vessel shrinkage (14).
Additionally, as research progressed within this field, it was found that not all side branch lesions require intervention. An important study by Koo et al. examined the hemodynamic significance of these lesions. They performed FFR (fractional flow reserve) in a subset of coronary side branch bifurcation lesions that had been “jailed” after bifurcation stenting. They found that of those lesions that they examined that were of 90% severity or greater, only 14 out of 25 had FFR values <0.75, demonstrating the fact that angiography is imperfect in being able to predict the actual hemodynamic importance of residual side branch lesions. Further, these data suggest that stable lesions that are less than 90% angio-graphically may be lesions that can be left alone after bifurcation stenting (provided that they have TIMI 3 flow) (15).
BIFURCATION CLASSIFICATIONS
It is important to accurately define what constitutes a bifurcation lesion, as the failure to do so has caused much of the controversy and uncertainty involving the trials that have attempted to study this topic. In 2007 a consensus group proposed the following definition: a bifurcation was “a coronary artery narrowing occurring adjacent to, and/or involving, the origin of a significant side branch.” (16) A significant side branch is defined by this group as a “branch that you do not want to lose in the global context of a particular patient (symptoms, location of ischemia, branch responsible for symptoms or ischemia, viability, collateralizing vessel, left ventricular function…).”
Once the definition of bifurcation stenting is established, the next important question is: what defines what are the main branch and the side branch? Louvard et al. (16) propose two approaches. The first they term the “nosologic approach,” where the left anterior descending (LAD), Cx, and posterior descending artery, regardless of their size, are always considered the main branch. The second approach they propose relates to quantitative coronary an-giography, where the main branch is the largest distal branch and the side branch is smaller.
There are currently seven major classification schemes for bifurcation lesions (17). The most commonly used both in clinical practice and in the trial literature is the Medina classification (18) (Fig. 23-1) likely due to its ease of use and ability to remember it. The Medina classification system is based on three number binary notations. Its focus is primarily on lesion location assigning a value of 1 or 0 based on the presence or absence of a lesion. A lesion is defined angiographically for this classification as being significant if it is 50% or greater. It then examines three positions: the main branch proximal to the side branch, the main branch distal to the side branch, and the side branch itself. As an example, a bifurcation that involved lesions in the main branch proximal to the side branch as well as the side branch itself would be coded as a 1,0,1 (see Figs. 23-2 to 23-5 for further examples).
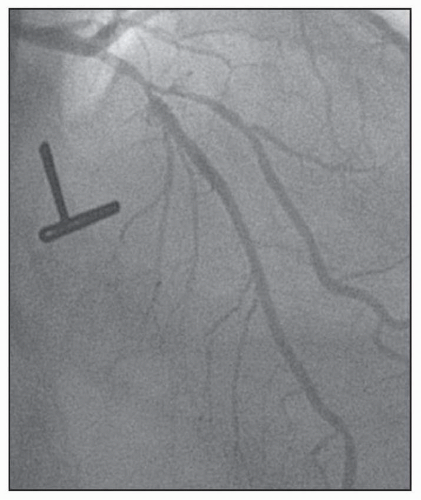 FIGURE 23-2 Medina classification 1,1,1, in which the proximal and distal main branch, as well as the side branch are involved in the lesion. (Image courtesy of Dr. Issam Moussa.) |
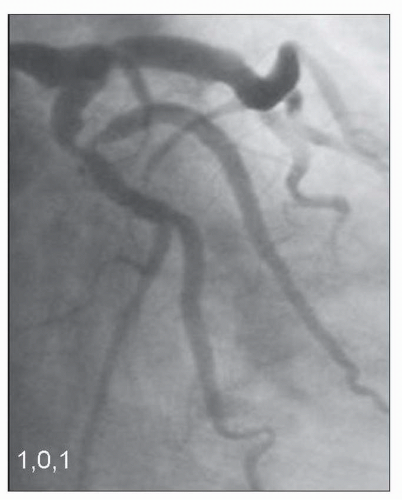 FIGURE 23-3 Medina classification 1,0,1, in which primarily the proximal main branch and side branch are involved in the lesion. (Image courtesy of Dr. Issam Moussa.) |
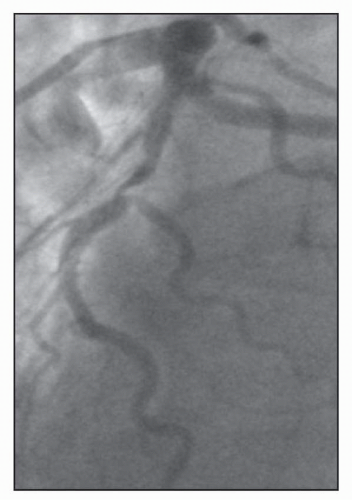 FIGURE 23-4 An additional example of Medina Classification 1,0,1. (Image courtesy of Dr. Issam Moussa.) |
However, as easy as this classification is to apply, it has limitations. Perhaps the greatest problem is that it has no way to stratify the overall prognostic importance of the side branch. As an example, it has been demonstrated that side branch occlusion is more common in a long side branch lesion as compared with a short one (19). Further, as pointed out by Colombo et al., the more important issue is the plaque burden surrounding the bifurcation as
well as the angle of the side branch in relation to the main branch. As Colombo states, bifurcations that are more Y-shaped and have angles <70° make wire access to the side branch more favorable, but also create a greater risk of plaque shift into the side branch. However, those lesions that are more T-shaped with an angle >70° can be tougher to wire, but the overall risk of plaque shift is lower (20). Additional studies reiterate the issue of the side branch angle being an overall predictor of procedural success and outcomes (21).
well as the angle of the side branch in relation to the main branch. As Colombo states, bifurcations that are more Y-shaped and have angles <70° make wire access to the side branch more favorable, but also create a greater risk of plaque shift into the side branch. However, those lesions that are more T-shaped with an angle >70° can be tougher to wire, but the overall risk of plaque shift is lower (20). Additional studies reiterate the issue of the side branch angle being an overall predictor of procedural success and outcomes (21).
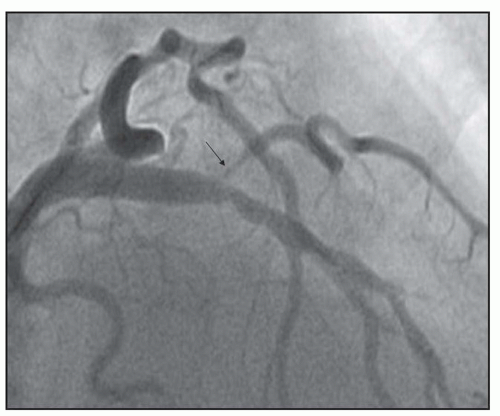 FIGURE 23-5 Medina classification 0,1,1, in which primarily the side branch and distal main branch are involved in the lesion. |
To date, all classification schemes, and in particular the Medina classification scheme, have difficulty giving full clinical importance to the different issues surrounding side branch lesions. These include issues such as angle and length, as mentioned above, in addition to how big the side branch is and what importance it holds to a particular territory (both myocardial size and how it provides collaterals) (17).
Separate from the classification scheme is the fact that the concept of a “true bifurcation” according to the Medina classification used in many trials is either 1,1,1; 1,0,1; or 0,1,1. However, not all trials were this strict in the lesions they included. Additionally, long side branch lesions were often not included in the trials.
STENTING METHODS
Some of the various stent methods outlined below have come in and out of vogue as newer trials have attempted to shed light on this topic.
V Stenting
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree