The present post hoc analysis of data from the COMBination of prescription Omega-3 with Simvastatin (COMBOS) study investigated the predictors of the low-density lipoprotein (LDL) cholesterol response to prescription omega-3 acid ethyl ester (P-OM3) therapy in men and women with high (200 to 499 mg/dl) triglycerides during diet plus simvastatin therapy. Subjects (n = 256 randomized) received double-blind P-OM3 4 g/day or placebo for 8 weeks combined with diet and open-label simvastatin 40 mg/day. The percentage of changes from baseline (with diet plus simvastatin) in lipids was evaluated by tertiles of baseline LDL cholesterol and triglyceride concentrations. The baseline LDL cholesterol tertile was a significant predictor of the LDL cholesterol response (p = 0.022 for the treatment by baseline tertile interaction). The median LDL cholesterol response in the P-OM3 group was +9.5% (first tertile, <80.4 mg/dl), −0.9% (second tertile), and −6.4% (third tertile, ≥99.0 mg/dl). Non–high-density lipoprotein cholesterol, very-low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride responses did not vary significantly by baseline LDL cholesterol tertile. The reductions in very-low-density lipoprotein cholesterol concentrations were greater than the increases in LDL cholesterol, where present, resulting in a net decrease in the concentration of cholesterol carried by atherogenic particles (non–high-density lipoprotein cholesterol) in all baseline LDL cholesterol tertiles. In conclusion, these results suggest that the increase in LDL cholesterol that occurred with the addition of P-OM3 to simvastatin therapy in subjects with mixed dyslipidemia was confined predominantly to those with low LDL cholesterol levels while receiving simvastatin monotherapy.
Numerous studies have shown that the long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid are effective for lowering triglycerides (TG) and non–high-density lipoprotein (HDL) cholesterol, when used alone or as an adjunct to statin therapy, in subjects with hypertriglyceridemia or mixed dyslipidemia. However, omega-3 fatty acids can also produce an increase in low-density lipoprotein (LDL) cholesterol in some patients. The COMBination of prescription Omega-3 with Simvastatin (COMBOS) study was an 8-week, placebo-controlled, double-blind trial. In the COMBOS study, 4 g/day prescription omega-3 acid ethyl esters (P-OM3) combined with 40 mg/day simvastatin significantly lowered the concentrations of TG, very-low-density lipoprotein (VLDL) cholesterol, and non–HDL cholesterol and increased the HDL cholesterol in patients with mixed dyslipidemia. During treatment, a trend was seen for an increase in LDL cholesterol with P-OM3 relative to placebo (+0.7% vs −2.8%, p = 0.052). The present post hoc analysis of data from the COMBOS study was undertaken to examine the relation between the baseline concentrations of LDL cholesterol and TG and the LDL cholesterol response to P-OM3 when combined with simvastatin therapy.
Methods
A full description of the procedures and main results from the COMBOS study have been previously published. In brief, the participants were men and women 18 to 79 years old who had been receiving stable-dose statin therapy for ≥8 weeks at study enrollment. The subjects completed an 8-week lead-in of the National Cholesterol Education Program Therapeutic Lifestyle Changes diet plus 40 mg/day simvastatin therapy (Zocor, Merck, Whitehouse Station, New Jersey). After the lead-in phase, those who had a fasting TG concentration of 200 to 499 mg/dl and LDL cholesterol concentration of ≤10% above their National Cholesterol Education Program Third Adult Treatment Panel treatment goal were eligible to enter the double-blind treatment phase. During the treatment phase, the patients underwent 8 weeks of the Therapeutic Lifestyle Changes diet and received 40 mg/day simvastatin therapy combined with either 4 g/day P-OM3 (Lovaza, previously known as Omacor, GlaxoSmithKline, Philadelphia, Pennsylvania) or placebo.
The prespecified primary outcome variable was the non-HDL cholesterol concentration (calculated as the difference between the total cholesterol and HDL cholesterol concentrations). Additional outcome variables included the levels of TG, VLDL cholesterol (calculated), LDL cholesterol (direct), and HDL cholesterol. The fasting serum lipoprotein lipids were analyzed by the Mayo Central Laboratory for Clinical Trials (Rochester, Minnesota), as previously described.
Statistical analyses were generated using Statistical Analysis Systems for the personal computer, version 8.0 or greater (SAS Institute, Cary, North Carolina). The baseline lipoprotein lipid values were calculated as the average of the values collected at weeks −2, −1, and 0. The end-of-treatment values were defined as the average of those collected at weeks 6 and 8, with the last observation carried forward (nonbaseline values only) for the subjects with missing end-of-treatment data.
Two-way analysis of variance was used to assess the relations between the baseline levels of LDL cholesterol and the TG and lipid responses. Each analysis of variance model used the percentage of change from the baseline LDL cholesterol as the dependent variable and contained terms for treatment group, baseline LDL cholesterol or TG tertile, and treatment by baseline tertile interaction as independent variables. As with the primary analysis reported in the COMBOS study, the analyses were based on ranks because of the significant non-normality for several variables.
Results
In the double-blind study, 256 subjects were randomly assigned to receive P-OM3 (n = 123) or placebo (n = 133), and 254 were included in the modified intent-to-treat analyses. The subjects were predominantly men (57.5%) and non-Hispanic white (95.7%) and had a mean age of 59.8 years.
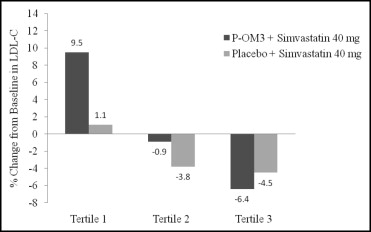
No treatment by tertile interaction was present for the baseline tertiles of TG (data not shown). The lipoprotein lipid concentrations at baseline and the responses according to the baseline LDL cholesterol tertile are listed in Table 1 . The baseline LDL cholesterol tertile had a significant interaction with treatment for the LDL cholesterol response (p = 0.022). The percentage of changes in LDL cholesterol from baseline to the end of treatment according to treatment group and baseline LDL cholesterol tertile are shown in Figure 1 . The largest increase relative to placebo in the LDL cholesterol concentration occurred in the group of subjects with the lowest baseline LDL cholesterol and was offset by a larger decrease in the VLDL cholesterol concentration ( Table 1 ), resulting in a net reduction in non-HDL cholesterol in all tertiles ( Table 1 ).
| Lipid Parameter | LDL Cholesterol (mg/dl) | |||||
|---|---|---|---|---|---|---|
| <80.4 | 80.4–<99.0 | ≥99.0 | ||||
| P-OM3 (n = 43) | Placebo (n = 41) | P-OM3 (n = 40) | Placebo (n = 46) | P-OM3 (n = 39) | Placebo (n = 45) | |
| Very-low-density lipoprotein cholesterol | ||||||
| Baseline (mg/dl) | 52 (45, 60) | 54 (45, 62) | 53 (45, 59) | 50 (44, 60) | 51 (45, 58) | 50 (46, 57) |
| Change (%) | −27 (−39, −15) | −7 (−19, 1) | −28 (−36, −13) | −10 (−16, 2) | −29 (−41, −11) | −7 (−15, 8) |
| Non–high-density lipoprotein cholesterol | ||||||
| Baseline (mg/dl) | 112 (105, 127) | 113 (103, 126) | 138 (127, 146) | 138 (124, 148) | 153 (141, 169) | 159 (153, 181) |
| Change (%) | −5 (−14, 5) | 0 (−5, 6) | −13 (−19, −6) | −4 (−11, 4) | −11 (−16, −3) | −2 (−12, 8) |
| High-density lipoprotein cholesterol | ||||||
| Baseline (mg/dl) | 42 (35, 48) | 38 (36, 45) | 47 (41, 55) | 43 (39, 50) | 49 (43, 54) | 47 (42, 54) |
| Change (%) | 4 (0, 11) | −1 (−7, 5) | 2 (−4, 7) | −1 (−9, 6) | 4 (−3, 13) | −1 (−5, 2) |
| Triglycerides | ||||||
| Baseline (mg/dl) | 282 (230, 364) | 281 (227, 332) | 268 (224, 299) | 269 (222, 347) | 255 (220, 313) | 261 (233, 290) |
| Change (%) | −27 (−42, −16) | −8 (−18, 2) | −32 (−42, −18) | −5 (−18, 6) | −30 (−43, −14) | −6 (−14, 12) |
Results
In the double-blind study, 256 subjects were randomly assigned to receive P-OM3 (n = 123) or placebo (n = 133), and 254 were included in the modified intent-to-treat analyses. The subjects were predominantly men (57.5%) and non-Hispanic white (95.7%) and had a mean age of 59.8 years.
No treatment by tertile interaction was present for the baseline tertiles of TG (data not shown). The lipoprotein lipid concentrations at baseline and the responses according to the baseline LDL cholesterol tertile are listed in Table 1 . The baseline LDL cholesterol tertile had a significant interaction with treatment for the LDL cholesterol response (p = 0.022). The percentage of changes in LDL cholesterol from baseline to the end of treatment according to treatment group and baseline LDL cholesterol tertile are shown in Figure 1 . The largest increase relative to placebo in the LDL cholesterol concentration occurred in the group of subjects with the lowest baseline LDL cholesterol and was offset by a larger decrease in the VLDL cholesterol concentration ( Table 1 ), resulting in a net reduction in non-HDL cholesterol in all tertiles ( Table 1 ).



