Barrett’s Esophagus
Matthew J. Schuchert
James D. Luketich
Historical Perspective
The pathologic features of Barrett’s esophagus were first described in 1906 by the Boston surgeon Tileston,86 who evaluated a series of patients with “peptic ulcer of the esophagus” in autopsy specimens. He described a mucous membrane around the ulcer “similar to that found in the stomach.” He was also the first to suggest that this pathologic entity was associated with esophageal reflux, and weakening of the musculature at the level of the cardia (now known to be the lower esophageal sphincter).17 In 1950, Norman Barrett (Fig. 152-1) published his landmark treatise on chronic peptic ulcer of the esophagus and “oesophagitis.” Interestingly, he contended that “most of these cases are—in truth—examples of congenital short oesophagus in which a part of the stomach extends upwards into the mediastinum—or even to the neck—and that in this stomach a typical chronic gastric ulcer can form.”5 He suggested that these findings were distinct from those seen in reflux esophagitis. Bosher and Taylor11 were the first to recognize that this specialized epithelium contains intestinal-type goblet cells, now recognized as the histologic hallmark feature of Barrett’s esophagus. In 1952, Morson and Belcher56 described a case of adenocarcinoma arising in the setting of a columnar-lined esophagus. In 1953, Allison and Johnstone3 published a series of seven patients in whom the lower esophagus had a columnar lining and were the first to convincingly establish that the columnar-lined segment actually represented the esophagus, not the stomach.3 It was not until 1957 that Barrett6 agreed with this assessment. He postulated at that time that “it is probably the result of a failure of the embryonic lining of the gullet to achieve normal maturity.”17 In 1959, Moersch55 asserted that this lining did not represent congenital tissue but rather was the sequela of chronic reflux esophagitis. By the 1970s and 1980s, the associations between chronic gastroesophageal reflux disease, Barrett’s esophagus, and esophageal adenocarcinoma were well established.19,41,57
Definition and Histologic Features
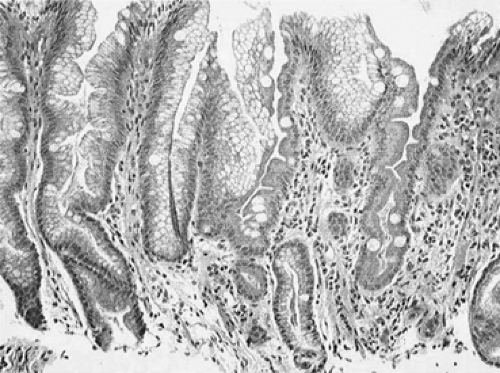
Barrett’s esophagus is currently defined as a change in the esophageal epithelium of any length that can be recognized at endoscopy and proven by biopsy to have metaplastic replacement of the normal esophageal squamous mucosa with columnar epithelium containing goblet cells71 (Fig. 152-2). There are three distinct subtypes of columnar metaplasia: fundic (indicated by the presence of parietal and chief cells), cardiac (indicated by the absence of parietal and chief cells), and intestinal (indicated by the presence of goblet cells). Combinations of these subtypes are not uncommon. Only the intestinal subtype is believed to carry a significant risk of malignant transformation.
For many years, the definition of Barrett’s esophagus included the criterion that the columnar mucosa must extend >3 cm above the gastroesophageal junction. However, it is now recognized that even short segments of Barrett’s esophagus can be associated with the development of esophageal adenocarcinoma.64 Although it seems logical that the risk of cancer should correlate with the extent of esophageal metaplasia, this notion remains unproven, and currently patients with long- and short-segment Barrett’s esophagus are managed similarly.20 The recently adopted Prague C & M grading system provides a means of standardizing the description of the extent of involvement by Barrett’s esophagus.77 Metaplastic epithelium is
described based on the length in centimeters of circumferential involvement (C) and maximum overall length (M). This grading system will help in standardizing the description of Barrett’s esophagus in clinical studies and everyday practice.
described based on the length in centimeters of circumferential involvement (C) and maximum overall length (M). This grading system will help in standardizing the description of Barrett’s esophagus in clinical studies and everyday practice.
Epidemiology
Barrett’s esophagus is most commonly diagnosed in patients with chronic gastroesophageal reflux disease (GERD).84 Approximately 2% to 4% of patients undergoing endoscopy for GERD symptoms will be found to have a columnar-lined esophagus with specialized intestinal epithelium.16 Barrett’s esophagus is considered a premalignant lesion with a 50- to 100-fold increased risk of cancer as compared with the general population.18 The risk of a patient with Barrett’s esophagus developing esophageal adenocarcinoma has been estimated to be 0.5% per year.76 Risk factors for the development of Barrett’s esophagus include male gender, Caucasian ethnicity, obesity, smoking history, age >50, and a >5-year history of reflux symptoms.27 The incidence of Barrett’s esophagus has increased dramatically over the last few decades, corresponding with the increased recognition and frequency of GERD. Whether the increased recognition of GERD and detection of Barrett’s esophagus is responsible for the dramatic rise in the incidence of esophageal adenocarcinoma over the same period remains unproven.
Pathophysiology
Barrett’s esophagus typically arises in patients with chronic gastroesophageal reflux. Increased acid exposures, decreased lower esophageal sphincter muscle tone, and impaired esophageal acid clearance have all been correlated with the development of this condition.84 Chronic distal esophageal acid inflammation leads to a variety of cellular changes, including loss of disaccharidase activity, low mucosal glutaminase levels, and altered levels of mucosal protein synthesis.66 In addition, a variety of intracellular pathways are altered by reflux in Barrett’s metaplasia, including MAP kinase activation80 and upregulation of COX-2 expression.37
Although gastric acid exposure is believed to play an impor- tant role in Barrett’s metaplasia, bile reflux may be more closely associated with the development of this condition.85 Bile acids have also been demonstrated to have significant carcinogenic potential. At high concentrations, bile acids induce damage to cell and mitochondrial membranes, release of reactive oxygen species, and mutation of tumor suppressor genes, such as p53.43 Chronic distal esophageal injury by both bile and gastric acid thus produces cellular changes that lead to a loss of normal squamous cells and their replacement with metaplastic columnar epithelium. Animal studies support the notion that regenerative processes in response to chronic injury result in the development of Barrett’s metaplasia.13,83
Barrett’s epithelium likely progresses through a metaplasia–dysplasia–carcinoma sequence.42 Metaplastic and dysplastic epithelia are frequently found adjacent to each other within pathologic specimens. In addition, progression from metaplasia to low-grade dysplasia to high-grade dysplasia (HGD) and ultimately to adenocarcinoma has been observed in individual patients.36 The molecular pathogenesis of Barrett’s esophagus and esophageal adenocarcinoma includes the accumulation of multiple genetic alterations over time. In Barrett’s esophagus, loss of heterozygosity of tumor suppressor genes—such as p53, adenomatous polyposis coli (APC), deleted in colorectal cancer (DCC), and MTS1 (p16)—is correlated with progression from metaplasia to dysplasia to cancer.68 Gene expression profiling studies have revealed differentially expressed gene clusters identifying Barrett’s esophagus as an early–intermediate oncogenetic stage in the development of esophageal adenocarcinoma. Interestingly, the gene expression clusters were distinct from clusters expressed in the development of squamous cell carcinoma.32,88 Extensive work is ongoing to determine whether tumor markers and gene profiles can help clinicians distinguish which patients with Barrett’s esophagus are more likely to progress to adenocarcinoma. Unfortunately, there are insufficient data at present to support the use of these markers/profiles in routine clinical practice.
Diagnosis
Most patients with Barrett’s esophagus have a long-standing history of heartburn and/or regurgitation. Less frequent symptoms include dysphagia, chest pain, hematemesis, and melena. The presence of symptoms, however, is an unreliable predictor of the development of Barrett’s esophagus A high index of suspicion must therefore be maintained in patients at increased risk for the development of Barrett’s esophagus.
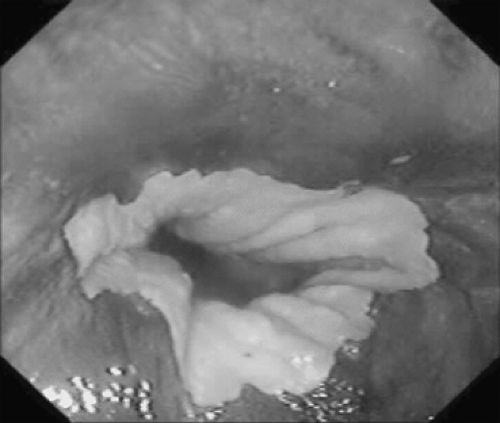
The diagnosis of Barrett’s esophagus is achieved by endoscopy, revealing abnormal salmon-colored columnar epithelium extending proximally within the esophagus (Fig. 152-3). As discussed above, the hallmark histologic finding within these specimens is the presence of columnar epithelial metaplasia with mucin-producing goblet cells (Fig. 152-2). It is important to note that detection of Barrett’s esophagus by standard esophagoscopy has a high false-positive rate and may accurately detect intestinal metaplasia in as few as 30% of patients. Endoscopic biopsy is therefore necessary to confirm the diagnosis. In a study of 570 patients undergoing upper endoscopy, columnar-appearing mucosa was identified in 146 patients, yet only 49 (34%) were found to have intestinal metaplasia.29 Similarly, another study of 474 patients with suspected Barrett’s identified intestinal
metaplasia in only 42% of the cohort.30 These studies highlight the shortcomings of conventional endoscopic assessment of patients suspected of having Barrett’s esophagus, with the greatest difficulty being encountered in short-segment disease. The introduction of novel endoscopic techniques for identifying Barrett’s esophagus and associated dysplasia may enhance diagnostic accuracy in patients at risk. These novel techniques include chromoendoscopy, fluorescence endoscopy, high-resolution endoscopy, and narrow-band imaging.
metaplasia in only 42% of the cohort.30 These studies highlight the shortcomings of conventional endoscopic assessment of patients suspected of having Barrett’s esophagus, with the greatest difficulty being encountered in short-segment disease. The introduction of novel endoscopic techniques for identifying Barrett’s esophagus and associated dysplasia may enhance diagnostic accuracy in patients at risk. These novel techniques include chromoendoscopy, fluorescence endoscopy, high-resolution endoscopy, and narrow-band imaging.
Chromoendoscopy employs staining agents such as methylene blue, Lugol’s solution, and indigo carmine during endoscopy to identify intestinal metaplasia and highlight epithelial regions that may contain areas of dysplasia or early cancer. These solutions are applied using a spray catheter, followed by water rinses to remove excess stain and allow visualization of the stained mucosa.22 Vital, or absorptive, stains (such as methylene blue and toluidine blue) are preferentially taken up by the intestinal mucosa, owing to its distinct absorptive properties; therefore areas of intestinal metaplasia are stained more intensely (Fig. 152-4). Contrast stains (such as indigo carmine) are not absorbed but highlight the surface of the mucosa, providing enhanced definition of mucosal lesions and patterns
(Fig. 152-5). Lugol’s solution is a mixture of iodine and potassium iodide and is absorbed by glycogen-containing squamous epithelium, resulting in a green–brown discoloration (Fig. 152-6). By contrast, areas of Barrett’s metaplasia do not stain with Lugol’s solution. Because of the stain’s ability to distinguish between squamous and columnar epithelium, it is useful in identifying lesions containing intestinal metaplasia and can assist in directed biopsy.
(Fig. 152-5). Lugol’s solution is a mixture of iodine and potassium iodide and is absorbed by glycogen-containing squamous epithelium, resulting in a green–brown discoloration (Fig. 152-6). By contrast, areas of Barrett’s metaplasia do not stain with Lugol’s solution. Because of the stain’s ability to distinguish between squamous and columnar epithelium, it is useful in identifying lesions containing intestinal metaplasia and can assist in directed biopsy.
Fluorescence endoscopy takes advantage of differences in tissue autofluorescence when stimulated by blue light (400–450 nm). Anatomic and structural variations in the underlying tissue architecture result in altered excitation patterns that can be used to localize mucosal abnormalities that might not be appreciable during standard, white-light endoscopy. Normal esophageal mucosa exhibits a predominantly green autofluorescence signal. Mucosal abnormalities (dysplasia, cancer) frequently result in a red–brown real-time spectral image (Figs. 152-7A and 152-7B). Preliminary studies have suggested that fluorescence endoscopy can increase the detection rate of high-grade dysplasia (HGD) or early cancers.38 The utility of fluorescence endoscopy in detecting early dysplasia and early cancers in Barrett’s esophagus has been evaluated in prospective randomized studies, with somewhat disappointing results. Kara and associates45 evaluated 50 patients with Barrett’s esophagus utilizing both white light and fluorescence endoscopy, performed by separate endoscopists, with a 4- to 6-week interval between procedures. The overall sensitivity for detecting HGD and early cancers was 62% in both groups, with a positive predictive value of 41% for white-light endoscopy versus 28% for fluorescence endoscopy. Acute inflammation contributed to a high false-positive rate (88%) among lesions detected by fluorescence endoscopy.45 Similar observations were seen by Borovicka and associates,10 who evaluated 187 patients undergoing endoscopic surveillance for Barrett’s esophagus. Autofluorescence evaluation detected 8 of 19 patients with proven HGD or early cancer in this cohort (sensitivity was 42%; positive predictive value was 12.1%).10 Third-generation fluorescence endoscopes are currently being
evaluated that use two separate charge-coupled device (CCD) chips for both standard endoscopic and autofluorescence imaging. Although the diagnostic accuracy of autofluorescence endoscopy as an isolated imaging modality may not be optimal, investigations are under way incorporating the information derived from this technique with other endoscopic modalities, such as high-resolution endoscopy and narrow-band imaging.25
evaluated that use two separate charge-coupled device (CCD) chips for both standard endoscopic and autofluorescence imaging. Although the diagnostic accuracy of autofluorescence endoscopy as an isolated imaging modality may not be optimal, investigations are under way incorporating the information derived from this technique with other endoscopic modalities, such as high-resolution endoscopy and narrow-band imaging.25
High-resolution endoscopy employs CCD chips capable of providing resolution of up to 1 million pixels that are fitted on the tip of the endoscope.44 In addition to superior resolution compared with fiberoptic endoscopes, high-resolution endoscopes are capable of lens adjustments that provide a variable focal distance and enable the endoscopist to zoom in on a particular region of interest.8 The enhanced magnification and resolution allow greater visualization of topographic mucosal detail as well as visualization of the mucosal microvasculature, thus permitting recognition of intestinal metaplasia.33 The irregular mucosal pattern of HGD is also discernible with this technique (Fig. 152-7C).78 The use of narrow-band imaging can enhance the mucosal contrast evident during high-resolution endoscopy and further facilitate recognition of distinct microstructural and microvascular patterns that correspond to areas of dysplasia. In a study of 50 patients with Barrett’s esophagus, 344 mucosal regions were assessed using high-magnification endoscopy with narrow-band imaging. Ten patients were identified with dysplasia. Endoscopy with narrow-band imaging revealed irregular microstructural and microvascular patterns predicting high-grade dysplasia, as confirmed by biopsies, with an overall sensitivity and positive predictive value of >90% (Fig. 152-8).4 In another study of 28 patients with Barrett’s esophagus, sensitivity for the detection of HGD or early cancer, as confirmed by biopsy, by narrow-band imaging was 86%.44 In summary, high-resolution endoscopy and narrow-band imaging are promising techniques that may improve the detection of early neoplasia without the need for chromoendoscopy in patients undergoing surveillance for Barrett’s esophagus. Further studies and technical refinements are necessary to determine the optimal use of these modalities in evaluating patients with Barrett’s esophagus. Although endoscopic methodology continues to improve,
none of the newer endoscopic methods discussed above have emerged as a “standard of care” in the diagnosis or surveillance of Barrett’s esophagus.
none of the newer endoscopic methods discussed above have emerged as a “standard of care” in the diagnosis or surveillance of Barrett’s esophagus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree