Takotsubo cardiomyopathy (TC) often occurs after emotional or physical stress. Norepinephrine levels are unusually high in the acute phase, suggesting a hyperadrenergic mechanism. Comparatively little is known about parasympathetic function in patients with TC. We sought to characterize autonomic function at rest and in response to physical and emotional stimuli in 10 women with a confirmed history of TC and 10 age-matched healthy women. Sympathetic and parasympathetic activity was assessed at rest and during baroreflex stimulation (Valsalva maneuver and tilt testing), cognitive stimulation (Stroop test), and emotional stimulation (event recall, patients). Ambulatory blood pressure monitoring and measurement of brachial artery flow–mediated vasodilation were also performed. TC women (tested an average of 37 months after the event) had excessive pressor responses to cognitive stress (Stroop test: p <0.001 vs baseline and p = 0.03 vs controls) and emotional arousal (recall of TC event: p = 0.03 vs baseline). Pressor responses to hemodynamic stimuli were also amplified (Valsalva overshoot: p <0.05) and prolonged (duration: p <0.01) in the TC women compared with controls. Plasma catecholamine levels did not differ between TC women and controls. Indexes of parasympathetic (vagal) modulation of heart rate induced by respiration and cardiovagal baroreflex gain were significantly decreased in the TC women versus controls. In conclusion, even long after the initial episode, women with previous episode of TC have excessive sympathetic responsiveness and reduced parasympathetic modulation of heart rate. Impaired baroreflex control may therefore play a role in TC.
The clinical syndrome of takotsubo cardiomyopathy (TC) has been well described, but the pathophysiological mechanisms underlying this transient myocardial process are still unclear. A number of mechanisms have been postulated, including microvascular disease, catecholamine-induced myocardial injury, multivessel coronary artery vasospasm, and plaque disruption in the major coronary arteries. However, the underlying reasons for susceptibility of the individual patient remain unknown. Several lines of evidence suggest that an exaggerated hyperadrenergic response to emotional or physical stimuli may be the underlying mechanism. Autonomic imbalance has been implicated in coronary vasospasm and microvascular coronary disease both of which are hypothesized mechanisms of TC, and catecholamine excess has been well documented in this condition. Comparatively little is known about cardiovagal (parasympathetic) function in TC. Reduced heart rate variability was demonstrated in the early acute phase, but the state of autonomic balance before or long after the event has resolved has not previously been studied. We tested the hypothesis that patients susceptible to TC have impaired baroreflex control, leading to inability to restrain and buffer sympathetic activation compared with controls.
Methods
From August 2010 to June 2013, we studied 10 women with a well-defined history of TC and 10 age-matched healthy women without the history of TC. Patients were recruited from our registry at New York University Langone Medical Center. Controls had no history of cardiovascular disease and were free of systemic and psychiatric illness. The study was approved by the Institutional Review Board of NYU School of Medicine. Informed consent was obtained from all participants.
Multiple measurements of sympathetic and parasympathetic activity were undertaken to evaluate autonomic cardiovascular function. Both physical (baroreflex-mediated) and mental (centrally mediated) stimuli were applied (see Figure 1 ). All measurements were acquired in the morning after an overnight fast in a quiet temperature-controlled autonomic laboratory. The autonomic testing procedures have been previously reported in detail.
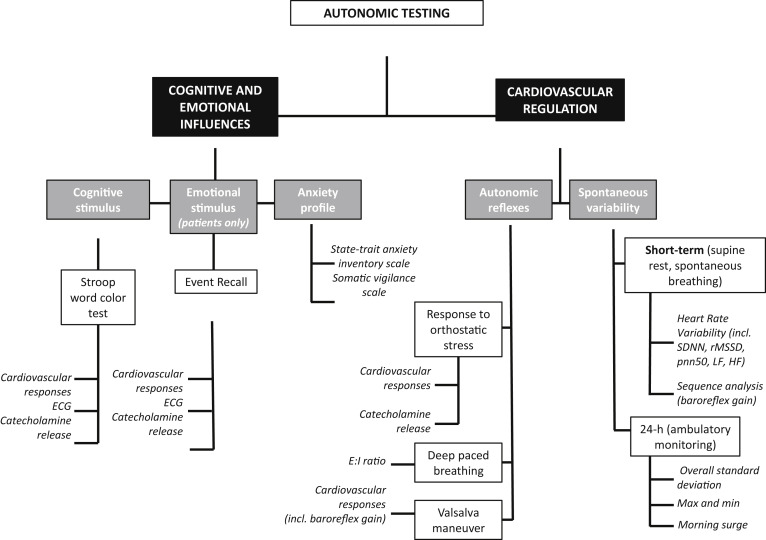
In brief, β blockers and other medications known to affect the autonomic nervous system were tapered and discontinued 5 half-lifes before testing. Participants were free of caffeine, nicotine, and alcohol from the previous evening. On arrival, an intravenous catheter was inserted into the right antecubital vein. Participants then completed the State-Trait Anxiety Questionnaire and Somatic Vigilance Scale. Static blood pressure (BP) measurements were obtained at 1-minute intervals on the left arm with an appropriate-sized cuff (Colin PressMate 880P). Continuous BP was measured using finger plethsymography (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands) with the hand supported at heart level. RR intervals were sampled at 500 Hz (PowerLab 16SP hardware and LabChart 7 software; AD Instruments, Dunedin, New Zealand).
The autonomic testing protocol is shown in Figure 1 and consisted of the following elements. RR intervals were recorded for 5 minutes during spontaneous breathing to assess heart rate variability. Heart rate and BP variability in the high-frequency (0.15 to 0.4 Hz) and low-frequency (0.04 to 0.15 Hz) bands were determined by means of spectral analysis. Time domain measurements of heart rate variability were also evaluated.
Additionally, subjects were guided to breathe at 6 breaths/min to evaluate expiratory-to-inspiratory ratio, a measurement of respiratory sinus arrhythmia modulated primarily by changes in parasympathetic outflow. Baroreflex function was assessed using the slope of the linear regression lines between beat-to-beat systolic BP (SBP) and RR interval changes at rest and during stimulation. Sequence analysis was performed on both “up ramps,” defined as 3 or more consecutive measurements with a sequential increase in both BP and RR intervals and “down ramps,” defined as 3 or more consecutive beats with decrease in both BP and RR intervals. Only sequences with significant correlations (i.e., R >0.8) were used in the analysis.
Participants performed a standardized Valsalva maneuver maintaining an expiratory pressure of 30 mm Hg for 15 seconds. Cardiovagal baroreflex gain was assessed by the relation between SBP and RR intervals during the Valsalva maneuver. BP overshoot was measured as the maximum change (from baseline) in SBP after release of the strain. The duration of the overshoot was measured as the time it took for the increase in SBP to subside (i.e., the first beat in which BP returned to prebaseline levels, see Figure 2 for examples).
After recovery, a 12-lead electrocardiogram was performed. The subject then performed a standardized Stroop color-word test for 5 minutes (Stroop effect app, version 1.1.0, Codeprincipals.com for Apple iPad). Toward the end, the 12-lead electrocardiogram was repeated. Subjects were then tilted upright to a 60-degree angle with footplate support for 10 minutes. After tilt testing, emotional stress was induced in patients by asking them to recall the precipitating events before their episode while monitoring hemodynamics and repeating the electrocardiogram.
Blood samples to measure plasma catecholamines were collected at rest, toward the end of the Stroop, after 10 minutes of upright tilt and during event recall (TC women only). Blood samples were kept cold, centrifuged and the plasma extracted before being assayed using quantitative high-performance liquid chromatography.
Ambulatory BP monitoring was performed with BP measurements at 20-minute intervals taken throughout the day and night (90207 monitor; SpaceLabs, Washington). Participants recorded sleep and activities in a diary. The morning surge in BP was defined as the difference between the average BP at night and the highest value obtained within 2 hours of awakening from sleep.
Brachial arterial reactivity was assessed in response to 5 minutes of distal artery occlusion produced by inflation of a forearm cuff to 50 mm Hg above SBP at rest. As described, brachial artery diameter was measured before and after cuff occlusion with a duplex ultrasound vascular imaging system connected to a 11-MHz linear array probe (SonoSite Inc., Bothell, Washington) and analyzed using computer-assisted edge detection image system (Artery Measurement Systems, Gothenburg, Sweden). Flow-mediated dilation was calculated as the percent change in the brachial artery diameter after forearm cuff release. Mean brachial artery blood flow velocity (cm/s) and peak shear rate (s −1 ) were also estimated.
Comparisons between groups and within groups after each stimulus were made using unpaired and paired t tests, respectively. Parametric and nonparametric evaluations were used as appropriate. Linear regression analysis with Pearson correlation coefficients was used to assess the relation between SBP and RR intervals. Individual baroreflex gain was calculated for each patient for comparison between groups. Data were analyzed using Prism, version 5.0, (GraphPad Software, Inc. 2011, La Jolla, CA). p Value <0.05 (2-tailed) was considered statistically significant.
Results
The clinical characteristics of the women during the acute takotsubo episode and at the time of testing are detailed in Table 1 . TC women were studied a median of 37 months after the episode (range 8 to 72 months). Five had an emotional trigger of TC and 5 had a physical trigger. Six of the patients presented with ST elevation on the electrocardiogram. Eight of 10 had nonobstructive coronary atherosclerosis at angiography. One had a muscle bridge in the mid left anterior descending coronary artery but no atherosclerotic disease. All 6 TC women who were taking β blockers were safely able to discontinue this medication before testing. None of the patients was taking a selective norepinephrine reuptake inhibitor or any other medication expected to influence autonomic function. Controls were well matched ( Table 2 ). Self-reported anxiety scores (TC: 51 ± 5; controls: 59 ± 6 points, p = 0.48) and somatic vigilance scores (TC: 18 ± 2, controls: 23 ± 4 points, p = 0.17) were similar in both groups. Supine SBP readings at rest tended to be greater in the TC women.
| Takotsubo Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Age at Diagnosis (years) | 83 | 59 | 55 | 70 | 68 | 63 | 44 | 63 | 67 | 45 |
| Hypertension | + | 0 | + | + | 0 | + | 0 | + | 0 | 0 |
| Hyperlipidemia | + | 0 | + | + | + | + | 0 | + | 0 | 0 |
| Diabetes mellitus | 0 | 0 | 0 | 0 | 0 | + | 0 | + | 0 | 0 |
| Smoker | Former | Never | Former | Never | Never | Former | Never | Former | Never | Never |
| Current beta blocker therapy | + | 0 | + | 0 | + | 0 | + | 0 | + | + |
| Precipitating factor & circumstances | Husband died | Near miss car accident | Bicycling up a hill | Atrial fibrillation ablation | Emotional: Husband died | Upsetting news & colitis | Fear of son being injured | Surgery | Pain | White water rafting |
| Months from Diagnosis to Testing | 49 | 41 | 24 | 72 | 55 | 53 | 54 | 8 | 57 | 8 |
| On admission | ||||||||||
| Presenting symptoms | Chest pain | Chest pain, palpitation, nausea | Chest pain, dyspnea | Chest pain | Dyspnea | Diarrhea, nausea, vomiting, palpitations | Chest pain, dyspnea | Dyspnea | Palpitation, syncope | Chest pain |
| Peak troponin I (ng/mL) | 3.61 | 3.61 | 2.33 | Not available | Not available | 1.78 | 2.21 | 0.06 | 2.72 | 8.90 |
| Chest x-ray | Normal | Normal | Not available | Not available | Pulmonary edema | Normal | Normal | Bilateral pleural effusions | Bilateral pleural effusions | Pulmonary edema |
| Echocardiography: | ||||||||||
| EF at diagnosis (%) | 28 | 33 | 45 | 35 | 40 | 40 | 47 | 25 | 25 | 35 |
| EF at recovery (%) | 65 | 50 | 60 | 60 | 70 | 65 | 60 | 60 | 65 | 65 |
| LVOT Obstruction | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | + | 0 |
| Cardiac Catheterization: | ||||||||||
| Non-Obstructive CAD | + | + | + | + | + | 0 | + | + | + | 0 |
| LVEDP (mmHg) | 29 | 32 | 24 | 22 | 42 | 15 | 17 | 12 | 30 | 38 |
| Variable | TC Women (n = 10) | Controls (n = 10) | P Value |
|---|---|---|---|
| Height (cm) | 161±2 | 161±3 | 0.99 |
| Weight (kg) | 69±6 | 66±9 | 0.84 |
| Spontaneous baroreflex sensitivity indices (ms/mmHg) | |||
| Sequence analysis – Up ramps | 5.2±1.4 | 9.2±1.3 | 0.01 |
| Sequence analysis – Down ramps | 6.5±1.4 | 14.3±2.7 | 0.03 |
| Brachial Artery Ultrasound | |||
| Resting diameter (mm) | 3.75±0.27 | 3.18±0.48 | 0.01 |
| Peak diameter (mm) | 3.84±0.24 | 3.33±0.47 | 0.01 |
| Flow-mediated vasodilation (%; median, IQR) | 2.59±3.74 | 4.90±3.28 | 0.18 |
| Resting brachial flow velocity (cm/s) | 8.1±2.7 | 12.2±6.7 | 0.09 |
| Peak brachial flow velocity (cm/s) | 39.7±9.8 | 38.9±19 | 0.91 |
| Peak Shear rate (s -1 ) | 427±116 | 507±304 | 0.50 |
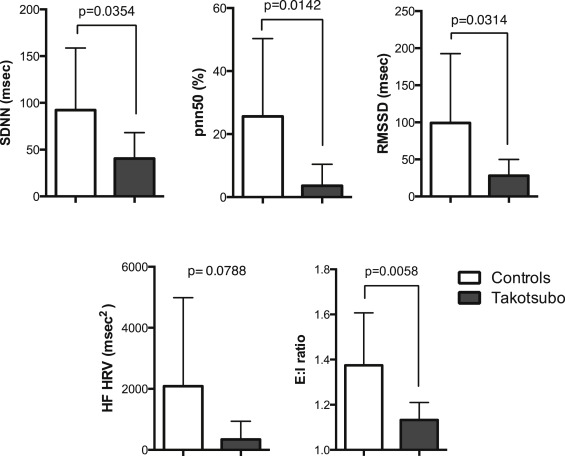
All participants were in normal sinus rhythm and supine heart rate was similar ( Table 2 ). Measurements of spontaneous heart rate variability were universally reduced in the TC women ( Figure 2 ), indicating less parasympathetic modulation of heart rate in the resting state. Paced deep breathing at 6 breaths/min induced smaller fluctuations in RR intervals and reduced expiratory-to-inspiratory ratios in the TC women ( Figure 2 ), again consistent with reduced parasympathetic activity in patients. There were no detectable differences in beat-to-beat BP variability at rest.
Spontaneous measurements of baroreflex gain were lower in patients with TC than controls, reflecting differences in the control of parasympathetic and sympathetic activity between the 2 groups at rest ( Table 2 ). During the Valsalva maneuver, both groups had a similar decrease in SBP in the straining phase (II; Table 3 ), indicating no defect in the activation of the sympathetic nervous system in response to a decrease in BP. After release of the strain, however, BP overshot was significantly more in the TC women, and the duration of the overshoot was more prolonged, suggesting excessive sympathetic activation during strain combined with delayed central restraint of sympathetic outflow after baroreceptor activation. Cardiovagal baroreflex gain during the Valsalva maneuver (measured over phases II to IV) was also significantly lower in the TC women compared with controls ( Figure 2 ).
| Variable | TC Women (n = 10) | Controls (n = 10) | P Value |
|---|---|---|---|
| Baroreflex mediated | |||
| Resting and upright tilt | |||
| Systolic BP (mmHg) | |||
| Initial supine | 150±8 | 135±4 | 0.08 |
| Relaxed supine | 137±5 | 125±4 | 0.43 |
| Upright tilt | 135±6 | 117 ±5 | 0.02 |
| Δ with tilt | -1±2 | -12±4 | 0.05 |
| Diastolic BP (mmHg) | |||
| Initial supine | 74±3 | 71±4 | 0.42 |
| Relaxed supine | 70±3 | 66±3 | 0.43 |
| Upright tilt | 73±3 | 66±3 | 0.43 |
| Δ with tilt | -1±2 | -1±3 | 0.91 |
| Heart rate (bpm) | |||
| Initial supine | 64±4 | 60±4 | 0.19 |
| Relaxed supine | 61±4 | 56±3 | 0.30 |
| Upright tilt | 75±5 | 65±4 | 0.14 |
| Δ RR intervals with tilt (msec) | -70±46 | -57±24 | 0.81 |
| Plasma norepinephrine (pg/ml) | |||
| Relaxed supine | 482±138 | 421±59 | 0.41 |
| Upright | 727±162 | 623±107 | 0.26 |
| Δ venous norepinephrine (%) | 71±16 | 51±12 | 0.61 |
| Plasma epinephrine (pg/ml) | |||
| Relaxed supine | 17±3 | 20±4 | 0.97 |
| Upright | 29±6 | 24±5 | 0.62 |
| Δ venous epinephrine (%) | 68±25 | 19±18 | 0.26 |
| Valsalva maneuver | |||
| Phase II | |||
| Expiratory pressure (mmHg) | 35±4 | 39±2 | 0.33 |
| Strain duration (sec) | 16±1 | 16±1 | 0.53 |
| Fall in systolic BP (mmHg) | -36±6 | -27±6 | 0.34 |
| Decrease in RR intervals (msec) | -144±49 | -84±43 | 0.37 |
| Phase IV | |||
| Max systolic BP overshoot (mmHg) | 31±6 | 10±3 | 0.004 |
| Duration of systolic BP overshoot (sec) | 24±4 | 11±1 | 0.007 |
| Slowing of RR intervals (msec) | +175±40 | 234±37 | 0.29 |
| Centrally mediated | |||
| Stroop Test | |||
| Δ Systolic BP (mmHg) | 42±4 | 27±3 | 0.03 |
| Δ Systolic BP Standard deviation (mmHg) | 13±2 | 8±1 | <0.01 |
| Coefficient of variation (mmHg) | 7±1 | 5±1 | 0.06 |
| Low frequency systolic BP variability (mmHg) | 26±4 | 11±3 | <0.01 |
| Δ Diastolic BP (mmHg) | 19±3 | 11±2 | 0.01 |
| Δ RR (msec) | -187±23 | -135±36 | 0.53 |
| Δ Venous norepinephrine (%) | -2±7 | -8±4 | 0.48 |
| Δ Venous epinephrine (%) | 68±30 | 58±33 | 0.53 |
| Event Recall | |||
| Δ Systolic BP (mmHg) | 63±8 | ||
| Δ Diastolic BP (mmHg) | 27±3 | ||
| Δ RR intervals (msec) | – 196±23 | ||
| Δ Venous norepinephrine (%) | 8±5 | ||
| Δ Venous epinephrine (%) | 56±29 | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


