Fig. 4.1
Cartoon of the heart demonstrating the atrioventricular conduction system with a left accessory connection. The P-wave and QRS complex are show short the indicating the impulse PR interval bypassing the AV node; also shown is the “delta wave” transcribing the slow anomalous ventricular activation and resulting in the early arrival (preexcitation of the ventricle) of the impulse through the accessory pathway. [Reprinted Kuilig J, et al. Wolff-Parkinson White syndrome and accessory pathways. Circulation 2010;122(15): e480–e483. With permission from Wolters Kluwer Health]
Manifest Accessory Pathways
Accessory pathways may be manifest or concealed. Manifest accessory pathways comprise antegrade activation of the ventricular myocardium through the accessory pathway and can “manifest” as preexcitation on an electrocardiogram during sinus rhythm (Figs. 4.1, 4.2, 4.3). This pattern is called the Wolff–Parkinson–White syndrome for the three physicians who described the syndrome in 1930—short PR interval, preexcitation (delta wave), paroxysmal tachycardia, usually first appearing in young persons. Even when ventricular preexcitation is present on the surface ECG during sinus rhythm, the mechanism of the tachycardia is usually orthodromic AVRT.
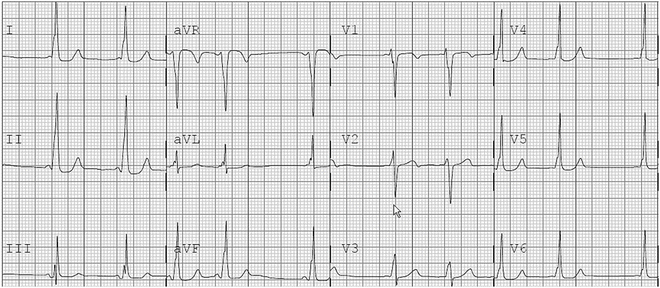
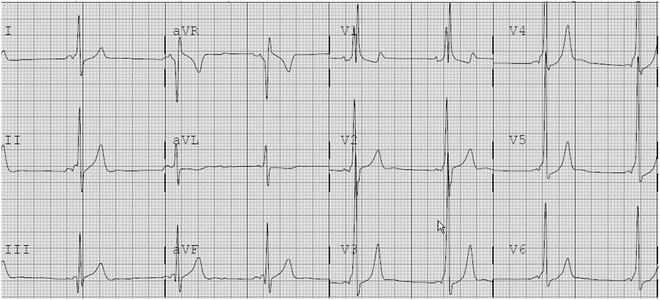

Fig. 4.2
12-Lead ECG demonstrating preexcitation with a right-sided pathway in an 8-year-old female. This was mapped to the right anterior septal area as suggested with the R/S transition between V2 and V3 (suggesting a possible septal pathway) and the positive preexcitation noted in II, III, aVF

Fig. 4.3
12-Lead ECG of patient with Wolff–Parkinson–White syndrome with a left posterior accessory pathway. The PR interval is short and delta waves are easily visualized. The R/S transition is before V1 suggesting a left-sided pathway
Concealed Accessory Pathways
Concealed accessory pathways are those that do not have antegrade conduction, but only
Definitions :
Orthodromic is used to describe accessory pathway (AP)-mediated tachycardias in which there is normal conduction from the atria to the ventricle via the AVS-HPS.
Antidromic is used to describe AP-mediated tachycardias which traverse the AP from atrium to ventricle and proceed backwards up the HPS.
Antegrade indicates conduction from atria to ventricle.
Retrograde indicates conduction from ventricle to atrium.
Concealed pathways only conduct in the retrograde direction.
Manifest pathways conduct in the antegrade direction with or without retrograde conduction as well.
Mechanism of Atrioventricular Reentrant Tachycardia
Atrioventricular reentrant tachycardia can be either orthodromic or antidromic. Orthodromic AVRT (Fig. 4.4), comprising approximately 90 % of AVRT, denotes antegrade conduction through the AVN-HPS and retrograde conduction through the accessory pathway, producing a narrow QRS tachycardia. This pattern can be seen in all patients with concealed and the great majority of patients with manifest pathways. If a patient has a manifest accessory pathway (WPW), ventricular preexcitation is no longer present during orthodromic AVRT as the antegrade ventricular activation occurs through the His-Purkinje system, no longer passing from the atrium to the ventricle across the accessory pathway.
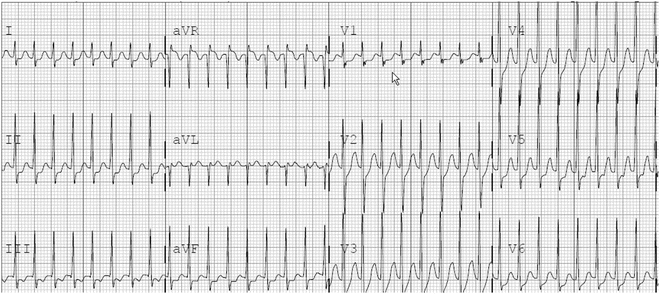

Fig. 4.4
12-Lead ECG with narrow QRS tachycardia in a 10-year-old female
In contrast, antidromic AVRT is characterized by antegrade conduction through a manifest accessory pathway (and not a concealed one) and retrograde conduction through the His-Purkinje-AV nodal system, resulting in a wide QRS complex tachycardia, mimicking ventricular tachycardia (Figs. 4.5a and 4.6). Activation of the ventricular myocardium via the accessory pathway results in a wide QRS complex secondary to working myocardial cell-to-cell conduction rather than conduction through the His-Purkinje system.
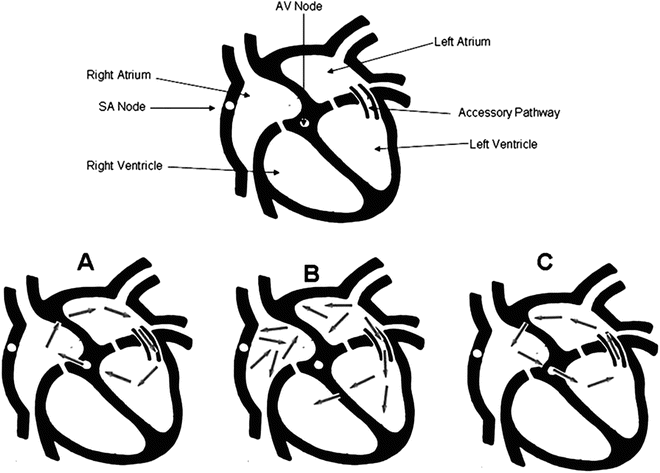
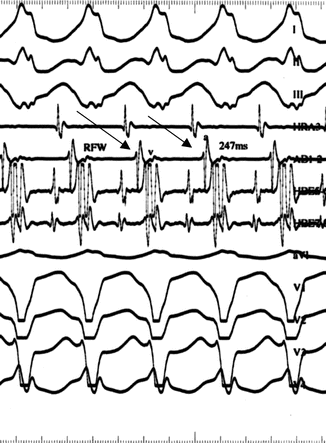

Fig. 4.5
Cartoon demonstrating typical orthodromic AVRT (C), rare antidromic AVRT (A), and antidromic AVRT during atrial fibrillation (B). (see text). [Reprinted Kuilig J, et al. Wolff–Parkinson–White syndrome and accessory pathways. Circulation 2010;122(15): e480–e483. With permission from Wolters Kluwer Health]

Fig. 4.6
An 8-Lead ECG demonstrating antidromic tachycardia in a 5-year-old boy. The top three tracings are recorded through the limb leads I, II, III; the bottom four tracings are aVL, V1, V2, V3, V4. The tracing fourth from the top is recorded through the high right atrial catheter. The tracing fifth from the top is recorded through the mapping catheter located in the right atrial free wall near the tricuspid valve. The sixth and seventh tracings are recorded through the His bundle catheter located at the low right atrial-septal area. Note the wide QRS tachycardia with maximal preexcitation of a left bundle branch configuration, compatible with a right-sided pathway. Note the tight AV relationship between the right atrial free wall electrogram (arrows) and the surface QRS complex (and intracardiac ventricular electrogram) indicating antidromic conduction down the right lateral accessory conduction to the ventricle. Retrograde conduction (not shown) supporting the tachycardia was through a left-sided concealed accessory pathway. Ablation of both pathways was successful
ECG Diagnosis
For those patients with manifest pathways, the ventricular preexcitation pattern in sinus rhythm can be helpful in determining the location of the accessory pathway. A number of algorithms have been developed to aid in pathway location with variable accuracy depending on the accessory pathway location. Briefly, if the QRS transition (R greater than S-wave) is after V3 in the precordial leads, the pathway is most likely on the right side; if it is at or between V2 to V3, it is most likely a septal pathway. If the transition is before V2, it is most likely on the left side. Using published algorithms, further analysis of the delta wave polarity in the ECG leads can identify the location of the accessory pathway even more precisely. In patients with a concealed accessory pathway, the resting ECG is of no diagnostic value to determine accessory pathway location.
An ECG obtained during SVT can be very helpful to indicate AVRT (vs. AVNRT) and occasionally to evaluate the location of the accessory pathway, especially when compared to an ECG obtained in sinus rhythm. Retrograde P-waves are typically present (typically with an R-P retrograde interval greater than 65–70 ms) in the ST segment or T-wave during AVRT, especially in Lead I, and suggests the diagnosis. This contrasts with a retrograde P-wave less than 40–60 ms in typical AVNRT. Table 4.1 summarizes the findings for manifest and concealed pathways characteristically seen on the surface electrocardiogram during either sinus rhythm or SVT.
Table 4.1
Summary of the findings for manifest and concealed pathways
Sinus | SVT | |||||||
|---|---|---|---|---|---|---|---|---|
Concealed | Manifest WPW | Mahaim (Manifest) | Concealed | WPW Orthodromic | WPW Antidromic | Mahaim | ||
Atriofascicular | Nodo-or fasciculoventricular | |||||||
Rate—beats/min | Within normal limits for age | Within normal limits for age | Within normal limits for age | Infants: 200–300; children: 160–250 | Infants: 200–300; children: 160–250 | Infants: 200–300; children: 160–250 | Infants: 200–300; children: 160–250 | Infants :200–300; children: 160–250 |
Rhythm | Regular | Regular | Regular | Regular | Regular | Regular | Regular | Regular |
P-wave | Normal | Normal | Normal | Absent or negative, within T-wave of lead II, aVF | Absent or negative, within T-wave of lead II, aVF | Absent | Absent | Absent or negative, buried within T-wave of lead II |
PR interval and QRS complex | Normal | Pre-excited (delta-wave, short PR interval) | Pre-excited (delta-wave, short PR interval) | Normal or wide when aberrantly conducted | Normal or wide when aberrantly conducted | Wide with QRS pattern similar to or wider than sinus rhythm | Wide (ART-AVRT) with LBBB QRS pattern | Wide (ORT-AVRT) with QRS pattern |
Manifest Accessory Pathways
Wolff–Parkinson–White Syndrome
The preexcitation is the result of a congenital accessory pathway that conducts antegrade reaching the ventricles in advance of activation through the AVN-HPS (Figs. 4.1, 4.2, 4.3). The incidence of Wolff–Parkinson–White syndrome in children has been estimated around one in every 1,000 individuals. Ventricular preexcitation can be quite subtle, occasionally apparent only as a lack of a q-wave in the lateral precordial leads due to slow conduction in the accessory pathway; accelerated conduction through the AV node (as is common in children); or in later accessory pathway activation of a left-sided pathway (the most frequent site of a pathway).
While both manifest and concealed pathways can mediate reentrant tachycardia, patients with Wolff–Parkinson–White syndrome have a greater rate of recurrent SVT than those without preexcitation. The clinical course of AVRT is age related. If diagnosed as an infant, the preexcitation may resolve to complete disappearance as a child grows, usually by 18–24 months of age (30 % recurrence if diagnosed less than 1 year of age). However, if preexcitation or documented SVT is seen after this age, AVRT is unlikely to self-resolve (94 % recurrence rate).
Furthermore, patients with WPW are noted to have a very small, albeit increased, risk for a sudden cardiac arrest. During atrial fibrillation, a conduction of the fibrillatory impulses may preferentially cross a fast conducting manifest accessory pathway, bypassing the more slowly conducting AV node resulting in a rapid life-threatening ventricular response (>250–300 bpm) (Fig. 4.5b; Chap. 20, Fig. 20.2). The incidence of sudden cardiac arrest in WPW patients has been estimated between one and 4.5 per 1,000 patient years, reflecting a higher incidence of atrial fibrillation in WPW patients. The highest prevalence of sudden cardiac arrest appears to be between the ages of 15 and 35 years. Patients with WPW syndrome who experience sudden cardiac death may have no history of a tachyarrhythmia. On the other hand, they may have had previous syncopal episodes. Potential risk factors for a sudden cardiac event with WPW include a younger age (<30 years), prior syncope or atrial fibrillation, male gender, familial WPW, or other heart disease.
The majority of accessory pathways are seen in patients with structurally normal hearts; however, approximately 10–15 % of patients with Wolff–Parkinson–White syndrome has congenital heart disease (Ebstein’s anomaly, l-transposition of the great arteries, cardiomyopathy, and intracardiac tumors). Six to nine percent of patients who have WPW syndrome and congenital heart disease are more likely to have multiple pathways. Approximately a quarter of patients with Ebstein’s anomaly also have preexcitation, the most common association. In addition, up to 43 % of patients with WPW and Ebstein’s anomaly have multiple pathways. In patients with other forms of congenital heart disease, 25 % had multiple pathways. Pathway location is also associated with congenital heart disease: right-sided pathways are more common in congenital heart disease (63 %) whereas left-sided pathways are more common in the structurally normal heart (61 %). Interestingly, the appearance of preexcitation in patients with congenital heart disease may be misleading, as some malformations—tricuspid atresia and hypoplastic left heart syndrome—may be associated with “pseudo-preexcitation” or the appearance of preexcitation and not have an accessory pathway.
The management of the asymptomatic patient with WPW has been addressed by the Pediatric and Congenital Electrophysiology Society in a recent consensus statement (2012). Patients who are 8 years of age or older and are found to have manifest preexcitation on an ECG without symptoms are recommended to undergo risk stratification, initially with noninvasive studies (i.e., exercise treadmill test; Holter monitor tracings). If inconclusive, invasive studies can be considered to determine those at higher risk for sudden cardiac arrest. In asymptomatic individuals under 8 years of age, the low risk of sudden death due to atrial fibrillation, allow conservative management and continued follow-up.
Atrioventricular and Mahaim Fibers
Mahaim fibers, as first described in 1938, are direct histologic links between either the AV node and the right ventricle (nodoventricular fibers) or the His bundle and the right ventricle (fasciculoventricular fibers). These nodo or fascicular ventricular fibers are anatomically common (though often inactive, i.e., not resulting in preexcitation or tachycardia) When they do produce ventricular preexcitation, it may be only in a “by-stander” roles and not participate in an arrhythmia reentrant circuit. In patients with a nodoventricular fiber, the PR interval varies relative to the fiber’s take-off from the node. In those with a fascicular-ventricular fiber arising from the His bundle and inserting into the ventricular myocardium the PR interval is normal. Rarely the nodoventricular fibers produce a wide QRS complex in the context of a different mechanism for SVT, such as AVNRT (Fig. 4.7), or even more rarely participate as a true antegrade limb of an atrioventricular circuit. Fasciculoventricular fibers have not been shown to participate in a reentrant circuit other than as a bystander. These bystander pathways are often best left alone and not ablated.
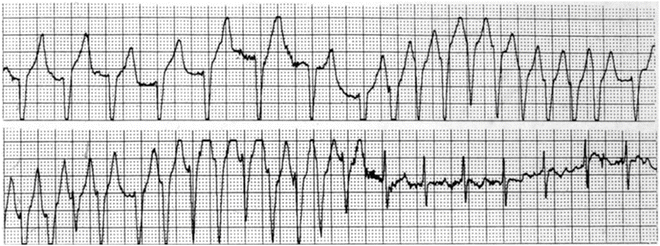

Fig. 4.7
Wide QRS rhythm and tachycardia followed by narrow QRS sinus rhythm in a 9-year-old girl. Electrophysiology study demonstrated a nodoventricular fiber (not involved in the reentrant circuit), which was ablated. Narrow QRS tachycardia was demonstrated post ablation of the Mahaim fiber and was found to be due to AVNRT. The slow pathway and arrhythmia were ablated
An uncommon right-sided atriofascicular fiber (somewhat confusingly also referred to as a Mahaim fiber) is located in the right posterior AV groove (Fig. 4.8). This accessory pathway has several features: (1) it typically conducts in only an antegrade direction producing preexcitation in a left bundle branch block configuration; (2) it inserts deep (not along the AV ring) in the right ventricle, often near the distal fascicle of the right bundle (moderator band); (3) the pathway demonstrates decremental conduction; (4) if present, the tachycardia is antidromic resulting in a wide QRS left bundle branch pattern SVT. These fibers were shown to be the cause of preexcitation characterized by left bundle branch block QRS morphology and wide QRS tachycardia. Intracardiac electrophysiologic studies demonstrate that the preexcitation and the wide QRS tachycardia are due to an atriofascicular accessory pathway coursing from the lateral right atrial tricuspid valve annular area to insert deeper in the right bundle rather than at the AV groove. As these fibers often have AV nodal properties, they may exhibit slow conduction velocity and “decremental conduction” with atrial extrastimulus and adenosine sensitive, resulting in AV block with adenosine administration. The hallmark of identifying the site for ablation is the identification of a fast action specialized conduction tissue electrogram distant in both location and timing from the His bundle electrogram, often near the moderator band. Ablation at that site terminates conduction within this pathway (eliminates the preexcitation), as well as the wide QRS tachycardia (Fig. 4.8). It can also be ablated on the right lateral annulus.
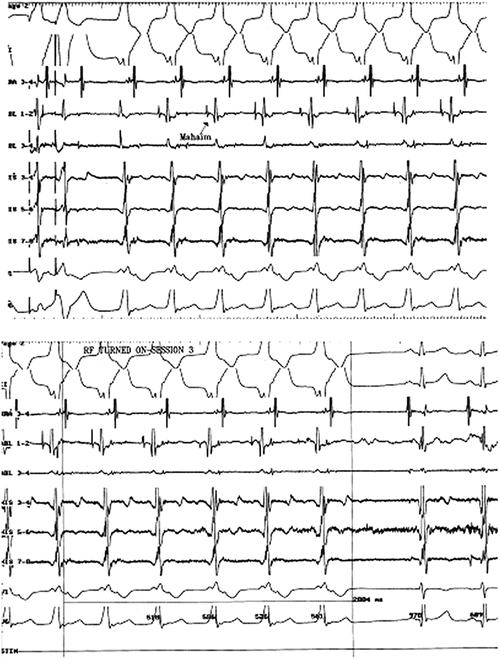

Fig. 4.8
Patient with an atrioventricular fiber causing wide QRS tachycardia. Top panel: Initiation of wide QRS tachycardia with antegrade conduction through the Mahaim fiber (note fast action deflection of atriofascicular fiber activation) and retrograde conduction through the normal His-Purkinje–AV node system. Bottom panel: Radiofrequency ablation of antegrade conduction in the atriofascicular fiber, terminating the tachycardia. Note the His bundle electrogram in the two sinus beats, as well as the absence of fast action deflection of the atrioventricular fiber in the mapping/ablation catheter post ablation
Diagnostic Evaluation of AVRT
Exercise Treadmill Testing and Holter Monitoring
Abrupt disappearance of ventricular preexcitation on the electrocardiogram during exercise suggests an accessory pathway effective refractory period perhaps as long as 360–390 ms, placing the patient in a lower risk category (Fig. 4.9). Exercise testing or Holter monitoring during activity has been used as a noninvasive method to evaluate the conduction properties of a manifest accessory pathway. Abrupt loss of preexcitation during exercise treadmill testing occurred in 15 % of a predominately s pediatric group of patients. However, in practice, disappearance of ventricular preexcitation exercise testing can be difficult to interpret due to movement artifact on the ECG and enhanced AV node conduction from increased adrenergic tone during exercise resulting in gradual (and less) not abrupt diminution of the ventricular preexcitation.
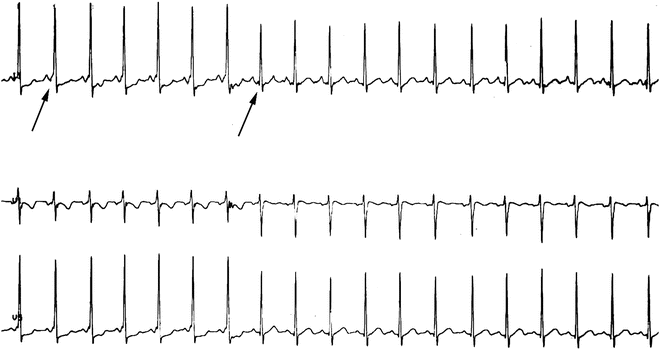





Fig. 4.9
Leads II, V1, and V5 during an exercise treadmill test. Note the delta wave in the onset of the QRS (left arrow) and abrupt loss over 2–3 beats (right arrow and next two beats) as the heart slightly increases, suggesting a slowly conducting accessory pathway
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


