Fig. 5.1
12-Lead electrocardiogram of AVNRT. Note that distinct P-waves are not visible
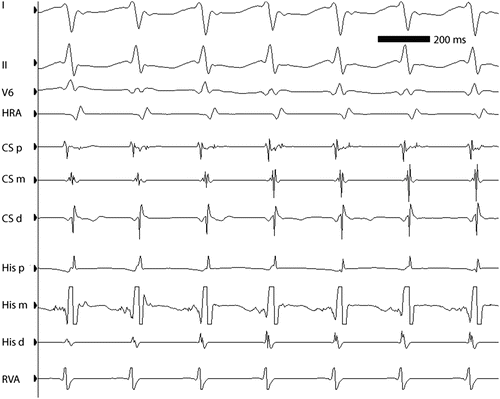
Fig. 5.2
ECG leads I, II, and V5 and intracardiac electrograms in a 13-year-old girl with AVNRT at approximately 260 bpm. Note the simultaneity of ventricular and atrial activation, characteristic of “typical” AVNRT. HRA high right atrium, CSp, CSm, CSd proximal, middle, and distal coronary sinus electrograms, respectively, HIS P, HIS M HIS D proximal, middle, and distal His bundle electrograms, respectively, RVA right ventricular apex
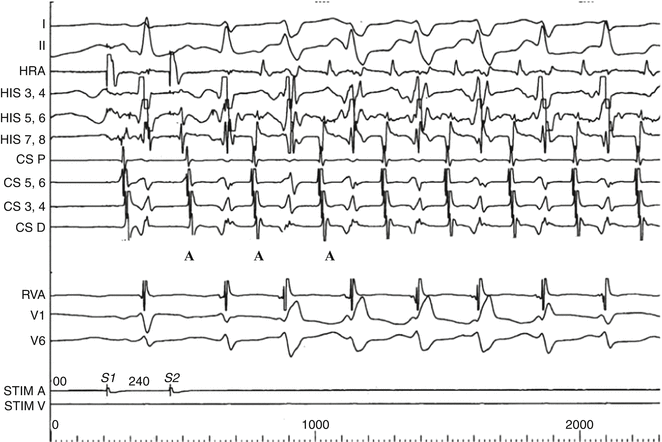
Fig. 5.3
“Atypical AVNRT” (slow–slow) initiated by programmed atrial stimulation in a 10-year-old girl. Note the long A2V2 and PR interval indicating slow conduction to the ventricle (third QRS complex from the left) and the echo beat (A) beginning the tachycardia. Note that the atrial echoes are not simultaneous with the QRS complex as in “typical AVNRT but are equidistant between the ventricular complexes. Note the rate-related right bundle branch bloc in the first four beats of the tachycardia followed by two narrow QRS complexes. The tachycardia cycle length does not lengthen during RBBB indicating that if there were an accessory pathway, it would not be on the right side (Chap. 4)
As in other forms of SVT, rate-related bundle branch-block may be seen, producing an “aberrant” wide-QRS tachycardia (Fig. 5.3) that is usually transient but can be mistaken for ventricular ectopy or tachycardia on ECG (Chap. 13). Depending on the patient’s autonomic state and individual AV node characteristics, the rate of typical AVNRT can range from 120 to 300 bpm, with rates typically 180–250 bpm.
Dual AV Node Physiology
The term “dual AV nodal physiology” denotes the underlying functional substrate required for AVNRT to occur. In dual AV node physiology, two functional conducting pathways exist between the atrium and the penetrating His bundle (Fig. 5.4). In the typical example, the antero-superior atrial connections (“fast” pathway) to the AV node comprise a pathway with a faster conduction velocity but a longer refractory period relative to the more slowly conducting postero-inferior atrial fibers (“slow” pathway), which have a shorter refractory period. As a result, an appropriately timed atrial premature beat (Fig. 5.4a) that fails to conduct through the refractory “fast pathway” may be successfully conducted through the AV node via the “slow pathway” (as it is not refractory, Fig. 5.4b). During the increased time which elapses as the impulse traverses the slow pathway, the fast pathway recovers excitability (no longer refractory) and is available for retrograde impulse propagation; the impulse also enters the His-Purkinje system activating the ventricles (Fig. 5.4c). In AVNRT, the atrium is therefore activated retrogradely at approximately the same time as the ventricle antegradely (Figs. 5.1 and 5.2). The reentrant circuit continues through atrial tissue, then antegradely through the slow AV nodal pathway and retrogradely through the fast, reactivating the atrium and ventricle with each cycle (Fig. 5.4d).
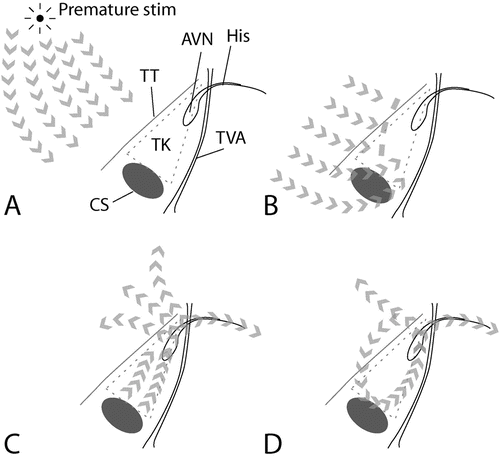

Fig. 5.4
Initiation of AVNRT by a premature atrial stimulus. (a) Atrial activation proceeds from the source of the premature stimulus. (b) The premature atrial activation approaches the AV node, but the anterior inputs (“fast pathway”) to the AV node are still refractory, and thus block conduction. (c) In contrast, the posterior inputs (“slow pathway”) to the AV node have a shorter refractory period, and are excitable. Here, conduction persists. The node is activated, leading to activation of the ventricle and (i.e., now the anterior aspect of the atrio-nodal junction is excitable) atrial tissues. (d) The reentrant circuit is established, with activation of atria and ventricles with each “lap” or revolution through the circuit. Abbreviations: AVN AV node, His His bundle, Stim stimulus, TK triangle of Koch, TT tendon of Todaro, TVA tricuspid valve annulus, CS ostium of the coronary sinus
Programmed atrial stimulation is the standard technique to demonstrate the presence of these functionally separate conduction pathways. As the coupling interval of the premature atrial extra-stimulus is progressively shortened, the septal atrial to His bundle (A-H) interval progressively lengthens, due to decremental conduction through the atrioventricular node (Fig. 5.5). At a critical coupling interval, the “fast” anterior conduction pathway is refractory. Conduction, however, persists through the slower posterior connections, resulting in an abrupt increase or “jump” in both the resultant A-H interval (defined conventionally as ≥50 ms or greater) and in the H1-H2 interval (Fig. 5.5), induced by only a 10 ms decrease in the premature coupling interval. This “jump” indicates a shift in conduction from the fast pathway to the slow pathway (i.e., dual AV node physiology) and fulfills two of the three conditions for reentry (unidirectional block in one pathway and slow conduction in the other pathway (Chap. 3). Single atrial echoes or multiple beats of tachycardia complete the reentry triad (recovery of excitability in the tissue of origin).
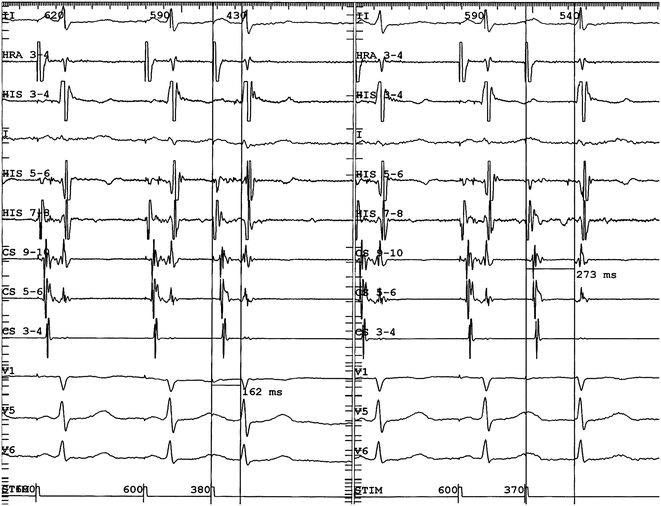

Fig. 5.5
Demonstration of dual AV nodal physiology at EP study. In the left panel atrial programmed extra-stimulation is performed using a drive cycle length (S1) of 600 ms and an extra-stimulus (S2) of 380 ms. The resultant A2-H2 was 162 ms as measured by the vertical caliper and the H1-H2 interval about 390 ms. In the right panel, the S1-S1 is again 600 ms but the S1-S2 is now 370 ms. The resultant A2-H2 is now 273 ms (and the H1-H2 interval increased by more than 100 ms) indicating conduction is blocked in the fast pathway but continues antegradely in the slow pathway
Although typical “slow–fast” AVNRT (Figs. 5.1 and 5.2) is the most common pattern seen with dual AV node physiology, variants exist. In the first variant, the so-called fast–slow form, the impulse travels antegradely in the fast pathway and retrogradely in the slow pathway (a reversed circuit compared to the typical form), transcribing a longer RP interval. A third infrequent variant has similar conduction velocity in both pathways of the reentry circuit (e.g., “slow–slow”) (Fig. 5.3). This form is characterized by a retrograde P-wave (negative in II, III, aVF) transcribed in the middle of the tachycardia cycle length. An important characteristic of the reentrant circuit of AVNRT is its independence from excitable tissues distal to the circuit, including the bundle of His and ventricles. It is therefore possible to observe AVNRT with 2:1 conduction to the ventricle (Fig. 5.6), which must be distinguished from atrial tachycardias.
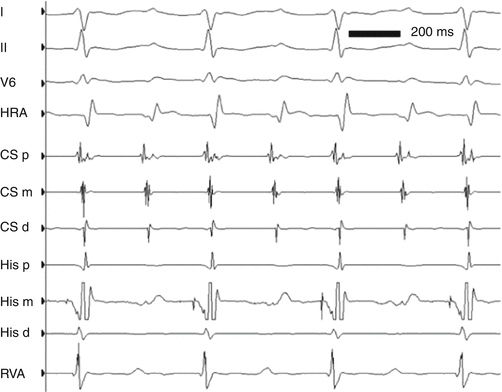

Fig. 5.6
The tracing demonstrates typical AVNRT, with a 2:1 A-V relationship between the atria and ventricles
On occasion atrial-programmed stimulation does not demonstrate dual AV node pathway physiology. Using ventricular extra-stimulation, retrograde conduction block occurs first in the fast pathway (longer refractory period) followed by conduction retrogradely through the slow pathway in the triangle of Koch and then antegrade conduction through the fast pathway, resulting in the “fast–slow” atypical form of AVNRT.
Noninvasive Treatment
As for all well-tolerated tachycardias, the decision to treat is largely governed by the findings of the individual patient. They include frequency and duration of episodes, intensity of symptoms with episodes, response to medications, and the patient’s and family’s views regarding the various options. Patients who have minimal symptoms with episodes that are short and self-terminating or that respond to vagal maneuvers may rightly pursue no further treatment. However, some patients in whom the response of the arrhythmia to vagal maneuvers is highly variable, or the symptoms are disruptive of activities of daily living elect treatment—either a trial of antiarrhythmic medication or ablation.
Because β-blockers are safe and available in convenient dosing (Table 5.1), they are the medication of first choice for many patients. This group of medications acts by reducing the excitability and probably also the conduction disparities of dual AV nodal pathways. Fatigue, malaise, and exercise limitations are common, even with the newer, more selective, and longer acting β1-blockers such as atenolol or nadalol, which cross the blood–brain barrier to a lesser degree. The young athlete taking β-blockers often reports fatigue during vigorous exertion; even the non-athlete may unilaterally discontinue β-blockers due to fatigue.
Table 5.1
Drugs used in the treatment of AVNRT
Drug | Class | Dose (mg/kg/24 h) | Dose interval (h) | Comment |
|---|---|---|---|---|
Digoxin | Glycoside | 8–10 mcg | 12 | Decreasing use, minimal antiarrhythmic effect |
Propranolol | β-Blocker | 1–4 | 8 | Depression, fatigue may occur |
Atenolol | β-Blocker | 0.5–1.5 | 12–24 | Fewer CNS effects than propranolol |
Diltiazem | Ca2+ channel blocker | 1.5–3.5 | 6–8 | Sustained-release form available |
Flecainide | Na+ channel blocker | 1–5 | 8–12 | Structural heart disease a contraindication |
Digoxin, at one time a common first-line therapy for SVT in children, is rarely used by pediatric electrophysiologists, except in infants without preexcitation. Its comparative efficacy remains debated, but digoxin may be preferable in the patient with a contraindication to β-blockers, such as asthma or prior unwanted side effects. Its use in the child with preexcitation on resting ECG is considered contraindicated, but in AVNRT it would not be expected to have significant risks.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


