Atrioventricular Nodal–Dependent Tachycardias and Preexcitation
Robert A. Schweikert
Douglas L. Packer
Overview
Atrioventricular (AV) nodal–dependent tachycardias comprise a specific subgroup of rapid, regular supraventricular arrhythmias that critically depend on conduction through the AV node for their perpetuation. The most common forms are AV nodal reentrant tachycardia (AVNRT) and AV reciprocating tachycardia (AVRT), the latter of which uses an accessory pathway (AP) as the retrograde limb of the circuit. The anatomic location around the mitral or tricuspid annulus of APs can be identified precisely at the time of electrophysiologic testing but can also be predicted based on the morphology of retrograde P waves during tachycardia or characteristic delta waves in those patients in whom these arrhythmias occur as a component of Wolff-Parkinson-White (WPW) syndrome. AV nodal–dependent tachycardias are typically initiated by premature atrial or ventricular complexes and have reasonably specific characteristics that allow their differentiation. Although both are short RP tachycardias, AVRT must have a ventriculoatrial (VA) or RP interval of at least 70 msec, whereas most AVNRTs have intervals of less than 60 msec, with retrograde P waves presenting as a pseudo-R wave in surface electrocardiogram (ECG) lead V1 during tachycardia. In some patients with APs, a reverse-direction tachycardia with anterograde conduction through the AP may occur, producing a maximally preexcited QRS complex on the surface ECG. Some patients with WPW syndrome are at potential risk for atrial fibrillation (AF) degenerating into ventricular fibrillation (VF). APs with unusual conduction characteristics contribute to the occurrence of preexcitation variants, including the permanent form of junctional reciprocating tachycardia (PJRT) and atriofascicular reentry. Because of their dependence on AV nodal conduction for their maintenance, AV nodal–dependent arrhythmias may be acutely terminated by vagal maneuvers or administration of adenosine or verapamil. Wide-complex tachycardias in these patients must be carefully approached without using drugs that accelerate AP conduction, which can lead to VF. Both pharmacologic and nonpharmacologic therapies may be applied in the long term for managing patients with these arrhythmias. Drug therapy may be directed at the AV node, the AP, or the initiating atrial or ventricular extrastimuli. Ablative therapy has also emerged as the mainstay of nonpharmacologic treatment of these arrhythmias.
Glossary
Anterior fibrous trigone
The region of convergent, dense, fibrous tissue of the posterior aortic and anterior mitral valve annuli.
Antidromic reciprocating tachycardia
A reverse-direction AV nodal–dependent tachycardia in which anterograde conduction occurs through the AP, whereas return retrograde atrial activation proceeds through the normal VA conduction system.
Atriofascicular Mahaim fiber
An atypical AP with AV node–like properties (or an accessory AV node) typically located in the anterior or anterolateral region of the tricuspid annulus. This pathway serves as a bridge between atrial and right bundle branch tissue.
Atriofascicular or Mahaim reentrant tachycardia
A preexcitation variant tachycardia that uses an atriofascicular fiber as its anterograde limb.
Atrioventricular nodal reentrant tachycardia (AVNRT)
An AV nodal–dependent tachycardia in which both anterograde and retrograde components of the reentrant substrate are located near or within the compact portion of the AV node.
Atrioventricular nodal–dependent tachycardia
A tachycardia that critically depends on conduction through the AV node for its perpetuation.
Atrioventricular reciprocating (reentrant) tachycardia (AVRT)
An AV nodal–dependent tachycardia with the AV conduction system as the anterograde and the AP as the retrograde limb of the tachycardia circuit.
Concealed accessory pathway
AP with retrograde-only conduction existing without evidence of anterograde ventricular activation through that pathway.
Discontinuous conduction
Characteristic of dual AV nodal physiology in which a more than 50-msec abrupt prolongation of AV nodal conduction time occurs with a 10-msec decrease in the coupling time of an atrial premature complex (APC), producing anterograde conduction through the AV node.
Dual atrioventricular nodal physiology
Electrophysiologic manifestation of two AV nodal pathways with different anatomic and/or physiologic properties that serve as a substrate for AV nodal reentrant tachycardia.
Koch’s triangle
A triangular space in the medial portion of the right atrium formed by the septal component of the tricuspid valve annulus, the coronary sinus orifice, and the tendon of Todaro. The AV node lies in the apex of this triangle.
Long RP tachycardia
Tachycardia with a retrograde P wave describing atrial activation occurring closer to the succeeding than the preceding QRS complex. These tachycardias have an RP interval that is more than 50% of the RR interval seen during tachycardia.
Permanent junctional reciprocating tachycardia (PJRT)
Preexcitation variant in which retrograde atrial activation occurs through an AP with nodelike or decremental electrical properties. These long RP tachycardias may be incessant and show negative P waves in leads II, III, and aVF.
Preexcitation
Early ventricular activation before that proceeding through the normal AV conduction system, as seen in patients with APs.
Short RP tachycardia
Tachycardia with a retrograde P wave describing atrial activation that occurs closer to the preceding than the succeeding QRS complex. These tachycardias have an RP interval that is less than 50% of the RR interval seen during tachycardia.
Ventriculoatrial interval
The VA interval beginning with the local ventricular activation and ending with earliest retrograde atrial activation. The surface ECG correlate is the RP interval from onset of the surface QRS to the onset of the retrograde P wave.
Introduction
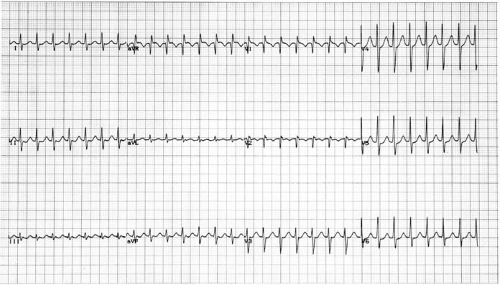
AV nodal–dependent tachycardias comprise a specific subgroup of rapid, regular supraventricular arrhythmias that critically depend on conduction through the AV node for their perpetuation. These arrhythmias typically use the normal AV conduction system as the anterograde limb of the reentrant circuit and therefore present with a narrow QRS complex on the surface ECG, as shown in Figure 65.1. Unlike atrial flutter or atrial tachycardias, which may continue despite the presence of high-grade AV block, AV nodal–dependent tachycardias are typically
terminated by vagal, drug, or nonpharmacologic interruption of AV nodal conduction.
terminated by vagal, drug, or nonpharmacologic interruption of AV nodal conduction.
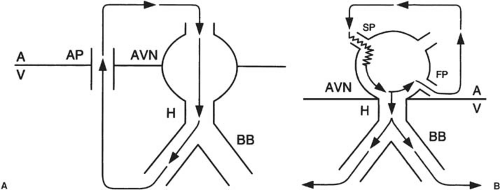
The most common of the AV nodal–dependent arrhythmias are AV nodal reentrant tachycardia (AVNRT) and AV reciprocating tachycardia (AVRT). AVNRT involves anterograde and retrograde components of the reentrant substrate that are near or within the compact portion of the AV node and neighboring bundle of His. AVRT involves the AV node and an AP as the limbs of the tachycardia circuit (Fig. 65.2).
Historical Perspective
These arrhythmias have been a focus of extensive clinical interest during the last 100 years and have prompted numerous studies to clarify the underlying electrophysiology. Kent provided an early description of what we now refer to as the normal AV conduction system (1). Mines provided the first description of reentry in the AV node in 1913, and his explanation of the possible physiologic mechanism of this arrhythmia is essentially what we now refer to as “dual AV nodal physiology” (2). In addition, Kent (3) identified anomalous muscular bundles connecting the atria and ventricles. Building on Kent’s observations, Mines (4) suggested that such a pathway may serve as a component of a possible reentrant circuit.
By the end of the first decade of this period, a variety of case reports documented the occurrence of relatively benign, rapid, regular tachycardias accompanied by palpitations (5,6,7,8). Wood et al. (7) reported a patient with a short PR interval and prolonged QRS complex who died during an attack of paroxysmal tachycardia. Serial histological sections of a portion of this patient’s AV groove showed three muscular connections between the right auricle and ventricle at the right lateral border of the heart. Wolferth et al. (5) hypothesized that such accessory connections capable of AV conduction may be responsible for both ventricular preexcitation during sinus rhythm and the paroxysms of tachycardia in these patients.
In 1930 the combination of paroxysmal tachycardias of this type along with a bundle-branch-block QRS morphology and a short PR interval were codified as the Wolff-Parkinson-White (WPW) syndrome (8). The existence of apparent dual AV nodal physiology as additional avenues of AV conduction was further suggested by others (9,10,11), and in 1963 Kistin postulated the existence of multiple AV nodal pathways in humans (12). Other investigators also subsequently described the possibility of triple or multiple AV nodal pathways (13,14,15,16,17).
The understanding of the rhythm disorders created by these connections took a giant leap forward with the advent of catheter-based electrophysiologic studies (18,19,20,21,22,23). By positioning multiple recording electrodes within the heart and using electrical stimulation techniques, it has been possible to elucidate the roles of the AV node and AP in supraventricular tachycardias (SVTs). Furthermore, this information has also expanded our understanding of macroreentrant arrhythmias in general. In this regard, the AV nodal–dependent SVTs with their discrete anatomic components can be viewed as the “schoolmaster” of reentrant arrhythmias. Since then, volumes have been written about the preexcitation syndromes, their accompanying SVTs, and AVNRTs (24,25,26,27). More recently, the introduction of more advanced computerized and optical mapping techniques with improved resolution has allowed more precise elucidation of arrhythmia substrates and mechanisms (28,29,30).
These tachycardias also provided the substrate for the first foray into nonpharmacologic therapy for arrhythmias. In the late 1960s, several institutions (31,32) attempted to interrupt APs as a definitive cure for SVT. The first successful division of such a pathway by Sealy (32,33) in 1968 permanently cured a North Carolina man of his recurrent palpitations, firmly established the reentrant nature of AP-dependent arrhythmias, and ushered in the era of nonpharmacologic management of cardiac arrhythmias. Since then, several thousand APs have been surgically interrupted (34,35,36,37,38,39).
Based on lessons learned from such surgical interruption of APs, drug therapy for these arrhythmias has been largely supplanted by development of closed-chest, catheter-based ablation of these reentrant circuits. Radiofrequency (RF) ablation was originally reported for APs in 1987 (40) and for the slow pathway for cure of AVNRT in 1992 (41). RF ablation has been further refined by a number of investigators (42,43,44,45,46,47,48) and applied to tens of thousands of patients with AVRT and other AV nodal–dependent tachycardias. In addition to RF, other energy sources for catheter ablation have been developed, such as cryothermy, laser, ultrasound, and microwave sources (49). For AV nodal–dependent tachycardias, the most extensive experience has been with cryothermy (50,51), and there has been limited experience with lasers (52). Other energy sources for catheter ablation, such as ultrasound and microwaves, have not yet been studied extensively for AV nodal–dependent
tachycardias. Because of the high success rates and low accompanying risk, catheter ablation has nearly replaced drug therapy as the mainstay of treatment for AV nodal–dependent arrhythmias. Each of these historic milestones marks important progress in both our understanding of these arrhythmias and the development of effective clinical management strategies.
tachycardias. Because of the high success rates and low accompanying risk, catheter ablation has nearly replaced drug therapy as the mainstay of treatment for AV nodal–dependent arrhythmias. Each of these historic milestones marks important progress in both our understanding of these arrhythmias and the development of effective clinical management strategies.
Atrioventricular Reciprocating Tachycardias and Accessory Pathways
Anatomic Considerations
The occurrence of an AV nodal–dependent reentrant tachycardia, like other classic reentrant arrhythmias, requires two anatomically distinct components or pathways to create the tachycardia circuit (4). By definition, the first is the AV nodal conduction system. Conduction in most AV nodal–dependent tachycardias travels anterogradely through this AV conduction system to produce the classic narrow QRS complex seen on the ECG.
APs involved as the retrograde limb of the AV reentrant tachycardia circuit are formed by minute, electrically conducting muscle bundles that transverse the AV groove. These pathways establish a nonnodal avenue for AV or VA propagation across the mitral and tricuspid annuli, which otherwise electrically isolate the atria from the ventricles. These fibers may cross the AV groove directly, proceed on an angle, or in some cases arborize with one or more branch points before insertion into either ventricular or atrial muscle (53,54,55,56).
With the exception of the region near the anterior fibrous trigone between the aortic and mitral valves, which is usually void of APs, these accessory AV connections may be distributed anywhere along the mitral or tricuspid annuli. As noted in Figure 65.3, surgical and catheter ablation studies have demonstrated that approximately 55% of these AV connections are located at the left free-wall region. Furthermore, 25% are in the posteroseptal region, 15% are in a right free-wall location, and 5% are in the anteroseptal region (35,36,37,38,43,45,47,48,57). In addition, 7% to 15% of patients may have more than one AP (23,43,45,47,48,58,59,60,61). More recently, a unique AP involving a connection between the right atrial appendage and right ventricle has been described (62,63,64,65), and due to its epicardial location, catheter ablation from an epicardial approach using percutaneous pericardial instrumentation has been necessary in some cases (63,64). This highlights the role of interventional electrophysiology in the advancement of our understanding of arrhythmia substrates.
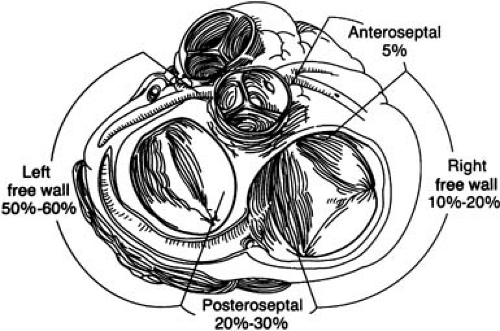 FIGURE 65.3. Distribution of accessory pathways (APs) around the mitral and tricuspid annuli. The left free-wall position is the most common AP location. |
Of all pathways described in reports of symptomatic patients, 2% to 3% conduct in an anterograde direction only, whereas 20% to 31% conduct in the retrograde/ventricular-to-atrial direction only (43,45,47,48,66). These connections are referred to as concealed APs. The mechanism of such concealment is further related to atrial and ventricular anatomy or the characteristic electrical properties of conduction through the pathway (25,67,68




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree