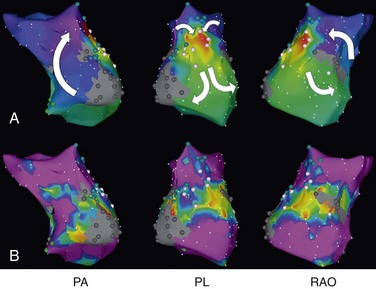
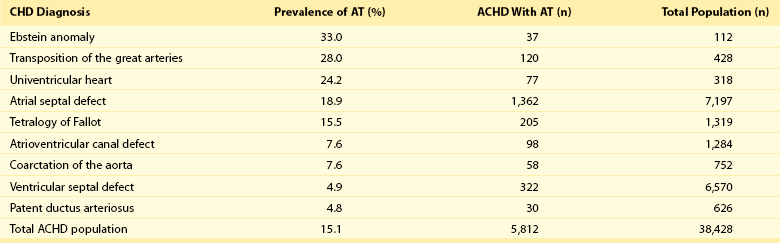
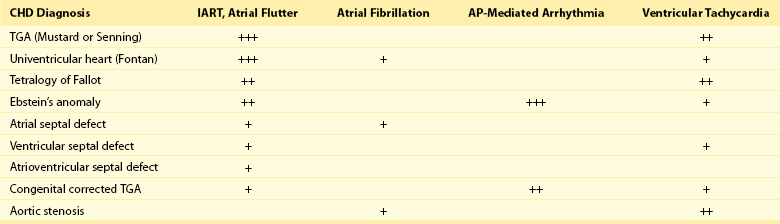
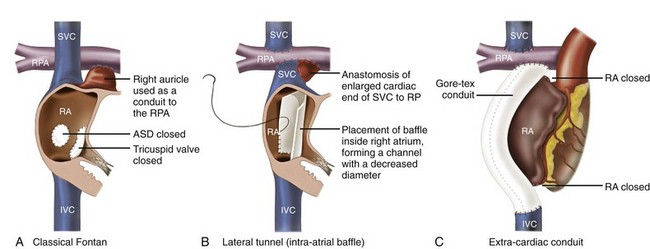
78 Advances in pediatric cardiovascular care have resulted in a rapidly expanding population of adults with congenital heart disease (ACHD). However, improved surgical outcomes in the young have resulted in alternate sources of morbidity and mortality in the adult, central among which are cardiac arrhythmias. Atrial tachyarrhythmias (ATs) are the most commonly encountered.1 The etiology of these arrhythmias is multifactorial, and they can arise as a consequence of underlying hemodynamic derangements, chronic cyanosis, elevated pressures, or prior surgery in which patches and suture lines create the substrate for tachycardia, most frequently macroreentrant. The clinical burden of these arrhythmias is substantial, and the development of AT is associated with an increased risk of stroke, heart failure, and death.1,2 Ventricular dysfunction and a predisposition to bradyarrhythmias can constrain the choice of antiarrhythmic agents in ACHD and as such, catheter ablation—coupled with or instead of antiarrhythmic therapy—is being used increasingly. The advent of sophisticated mapping systems has improved procedural success rates of catheter ablation,3 although recurrences after successful ablation occur more frequently in this patient cohort, motivating active and ongoing research in this area. This chapter presents an overview of the epidemiology of congenital heart disease (CHD) and reviews the clinical effects, mechanisms, and management of AT in this population. The development of advanced surgical techniques for patients with CHD and improved pediatric care have resulted in an increasing and aging population of ACHD, including those with complex lesions.1 More than 90% of children born with CHD are expected to survive to adulthood. In a large Canadian population-based database, the prevalence of CHD was 1.2% in children, 0.4% in adults, and 0.6% in the general population in the year 2000.4 For severe CHD lesions, the prevalence was 0.38 per 1000 adults, representing 9% of the total ACHD population. The prevalence of adults with severe CHD is rising faster than the prevalence of children with severe CHD and in the year 2000 there were nearly equal numbers of adults and children with severe CHD.4 Current estimates in the United States of people with CHD are in excess of 2 million with an approximate equal number of children and adults.5 It is expected that the number of adults with severe CHD will outnumber the number of children with severe CHD. These findings reflect the need for more specialized care facilities for ACHD patients to meet the needs of the additional morbidity burden including arrhythmias. A large population-based study demonstrated that the prevalence of AT is approximately 15% in ACHD, which is nearly threefold higher than in the general population.1 In general, older patients with CHD are more likely to develop AT. For example, the 20-year risk of developing AT is 7% in a 20-year-old patient and 38% in a 55-year-old patient.1 Overall, approximately 50% of 20-year-old patients with CHD will develop AT during their lifetime.1 This risk is higher in those with severe CHD lesions and in those with right-sided lesions.1,6 The prevalence and mechanism of arrhythmias varies according to the underlying anatomic defect and method of surgical repair (Tables 78-1, 78-2). The prevalence of AT ranges from 4.8% in patients with patent ductus arteriosus to 33% in those with Ebstein anomaly.1 The following section discusses the congenital cardiac malformations at highest risk of developing AT, including Ebstein anomaly, complete transposition of the great arteries (TGA), univentricular heart (UVH), atrial septal defect (ASD), and tetralogy of Fallot (TOF). Table 78-1 Prevalence of Atrial Tachyarrhythmias in Selected ACHD Cohorts Modified from Bouchardy et al: Atrial arrhythmias in adults with congenital heart disease, Circulation 120:1679-1686, 2009. Table 78-2 Relative Risk for Specific Tachyarrhythmias in Common Congenital Heart Defects Modified from Walsh EP: Interventional electrophysiology in patients with congenital heart disease. Circulation 115:3224-3234, 2007. Ebstein anomaly is an uncommon congenital cardiac malformation accounting for less than 1% of CHD lesions. It is characterized by apical displacement and tethering of the septal and posterior leaflets of the tricuspid valve, creating a broad area of atrialized right ventricle. There is often coexistence of an ASD (84%), but other cardiac anomalies can also occur, including pulmonary stenosis (10%) and ventricular septal defect (4%).7 The pathophysiologic abnormalities that occur in Ebstein anomaly place these patients at increased risk of developing AT: a markedly enlarged right atrium (RA), left-to-right atrial shunting, and the not infrequent occurrence of accessory pathways.8 A recent study from the Mayo Clinic demonstrated that 36% of operated patients reported an episode of AT in adulthood.9 In patients experiencing AT, the most common mechanism of AT is atrial fibrillation or flutter (64%), followed by atrioventricular reentrant tachycardia (AVRT) (45%) and atrioventricular nodal reentrant tachycardia (AVNRT) (9%).8 Approximately 17% of patients with AT demonstrate multiple mechanisms of AT.8 AVRT can induce atrial fibrillation, and in conjunction with rapid antegrade conduction over an accessory pathway this can in turn provoke ventricular fibrillation. Complete TGA is characterized by ventriculoarterial discordance and atrioventricular (AV) concordance. The prevalence of complete TGA is 5% to 7% of all congenital cardiac malformations. From the mid 1960s to the late 1980s, these patients underwent repair with atrial switch procedures using either artificial material (Mustard) or myocardial tissue (Senning) to redirect the systemic and pulmonary venous circulation, such that the morphologic right ventricle serves as the systemic ventricle and the morphologic left ventricle as the pulmonary venous ventricle. Although arterial switch surgery is the current procedure of choice, most adults currently have Mustard or Senning baffles. These surgeries involve extensive atrial reconstruction and predispose the patient to sinus node dysfunction and late AT.10 In a large cohort study of 478 early Mustard survivors (median age at operation, 1.3 years) the incidences of sinus node dysfunction and AT and were 60% and 24%, respectively, at 20 years after the index operation.10 Independent risk factors for the occurrence of late AT included perioperative bradycardia, permanent heart block, need for reoperation, and loss of sinus rhythm during follow-up. A later study in a subset of these patients demonstrated that by adulthood 48% of Mustard patients had experienced at least one episode of AT, with most patients having experienced intraatrial reentrant tachycardia (IART) or atrial flutter (AFL).11 Pulmonary hypertension, systemic ventricular dysfunction, and junctional rhythm before 18 years of age were independent predictors for AT.11 Documented AT has also been associated with sudden death in patients with atrial switch.12 The arterial switch operation is the most contemporary surgical approach for complete TGA. Besides restoring the morphologic left ventricle as the systemic ventricle, this procedure also reduces the incidence of sinus node dysfunction and late AT. A Japanese multicenter study of 624 1-year survivors (median age at operation, 2 months) demonstrated incidences of 1.0% and 4.3% for sinus node dysfunction and AT, respectively, during a mean follow-up duration of 10 years.13 This low incidence of AT appears to remain fairly constant during long-term follow-up, with a prevalence of approximately 4.5% reported at adulthood.14 These data support the notion that extensive atrial surgery is an important contributor to the development of AT in TGA patients. The Fontan palliation was introduced in 1971 to treat tricuspid atresia. Four decades later, the Fontan circulation encompasses a spectrum of anatomic substrates, staging options, and operative techniques resulting in one functional ventricular chamber.15 Types of Fontan procedures include the atriopulmonary connection, a right atrioventricular connection, and total cavopulmonary connection (i.e., an intracardiac or extracardiac lateral tunnel connection, or an extracardiac conduit; Figure 78-1). ATs are highly prevalent, depending on type of surgery and age, and are associated with substantial morbidity.16,17 Earlier single-center cohort studies, in which a large proportion of patients had an atriopulmonary Fontan, reported a higher incidence of late AT ranging from 16% to 22%, with a mean follow-up duration of approximately 5 years after surgery.18,19 Risk factors for the development of late postoperative AT included RA enlargement, systemic AV regurgitation, sinus node dysfunction, older age at the time of cardiac surgery, elevated preoperative pulmonary artery pressure, early postoperative arrhythmias, lower functional status, and longer follow-up duration.17–19 Earlier canine models of atriopulmonary Fontan have shown that suture lines alone can contribute to the development of IART. As a consequence of these observations, the Fontan procedure was revised to minimize both RA dilatation and the extent of atrial surgery, resulting in the (1) lateral tunnel connection and (2) extracardiac total cavopulmonary conduit. In a recent multicenter cross-sectional pediatric cohort study of 521 early Fontan survivors (mean age, 12 years) the prevalences of IART, AVRT, and focal AT were 7.3%, 1.8%, and 0.8%, respectively. The risk of IART decreased after the Fontan operation until 4 to 6 years after completion, and it then increased with age thereafter.17 The prevalence of IART was related to the amount of RA dilatation and extent of atrial surgery: 19% in the atriopulmonary connection, 7% in the intracardiac lateral tunnel, 5% in the extracardiac lateral tunnel, and only 2% in the external conduit Fontan.17 Previously, a single-center study demonstrated a 15-year incidence of late AT of 13% for patients with intracardiac lateral tunnel repair versus 39% for atriopulmonary Fontan patients.15 Intracardiac lateral tunnel repair requires a lot of atrial surgery, whereas with the extracardiac Fontan, there is less extensive atrial surgery. The use of an extracardiac Fontan circulation has resulted in a low incidence of AT, 4.2% during a mean follow-up of 6 years.20 Although the duration of follow-up for these newer surgical variants is shorter than for the atriopulmonary Fontan, it is hoped that the avoidance of an extensive atriotomy and intraatrial suture lines will contribute to a reduction of AT in the long-term. Figure 78-1 The different types of Fontan circulation. A, The atriopulmonary connection consists of closing the atrial septal defect (ASD) and connecting the right atrium (RA) directly to the right pulmonary artery (RPA). B, The intracardiac total cavopulmonary connection, or lateral tunnel procedure, connects the superior vena cava to the right pulmonary artery, so that the blood is directed from the inferior vena cava (IVC) to the pulmonary artery. C, The extracardiac cavopulmonary connection consists of a direct anastomosis of the superior vena cava (SVC) to the RPA and in the interposition of an extracardiac prosthesis between the IVC and the RPA. The advantage of the procedure is that it can be performed without myocardial ischemia, there are fewer suture lines in the RA, and there are no foreign material in the RA. (From d’Udekem et al: The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation 116:I157-I164, 2007.) Atrial septal defect is the most common congenital cardiac malformation in adults.4 Several anatomic types of ASD exist. Ostium secundum defects occur as a result of an increased resorption of the primum septum or an undergrowth of the flap of the foramen ovale, whereas ostium primum defects results from abnormal development of the endocardial cushions and are accompanied by anomalies of the mitral valve. The resultant atrial enlargement secondary to left-to-right atrial shunting—and mitral regurgitation in ostium primum defects—contributes to arrhythmia development. Sinus venosus defects result from the abnormal development of the sinus horn with a resulting defect in proximity to the sinus node. A shunt resulting from the ASD itself, anomalous pulmonary venous drainage, and prolonged AV node conduction predispose to subsequent arrhythmias. Secundum defects are the most common (60%), with primum defects accounting for 20% and superior sinus venosus defects 15%. In the absence of associated lesions, the development of late AT is low in those operated during childhood.21 Murphy et al.21 demonstrated that the incidence of late AT after closure increased from 17% in the 12- to 24-year-old age group to 55% in the over 41-year-old age group during late follow-up.21 The prevalence of AT is approximately 20% in patients undergoing surgical closure of ASD in adulthood, and the majority of patients with preoperative AT continues to experience AT during long-term follow-up after surgical closure.22,23 Furthermore, a randomized study showed that surgical closure at an older age (>40 years) does not appear to protect against new-onset AT compared with medical therapy.23 In the current era of predominantly percutaneous ASD closure, risk factors for the development of late AT after closure at adult age remain similar to those identified for the surgical cohort and include older age (>40 years) at time of closure and a prior history of AT.24 A prospective study of 200 patients with ASD demonstrated an incidence of AT of 16% during long-term follow-up after device closure.24 These data support the need to close defects at a young age to prevent the development of AT. Tetralogy of Fallot is the most common cyanotic heart disease in adults, and it accounts for approximately 10% of CHD lesions. Surgical repair includes patch closure of the ventricular septal defect, relief of right ventricular outflow tract obstruction, and repair of associated anomalies. Ventriculotomies have largely been abandoned in favor of transatrial or transpulmonary approaches to accomplish the repair, because the latter appear to reduce the risk for subsequent ventricular tachyarrhythmias (VT). Although VT is recognized as a potentially life-threatening arrhythmia in this patient population, the development of AT is an important contributor to morbidity—including heart failure, reoperation, subsequent VT, and stroke—and mortality.25 In a large multicenter cohort study of 793 patients with TOF, sustained AT and VT occurred in 10% and 12%, respectively, at 35 years after repair.26 Older age at repair, previous palliative surgery, and significant tricuspid regurgitation at follow-up were independent predictors of AT.26 In a recent multicenter, cross-sectional study (n = 556; mean age, 37 ± 12 years), the prevalence of AT was 20.1%.27 Interestingly, this study identified risk factors associated with a specific AT type. Independent factors associated with IART/AFL were RA dilatation and hypertension, whereas atrial fibrillation was associated with older age, left ventricular dysfunction, and left atrial dilatation. The number of cardiac surgeries was a risk factor for both IART/AFL and atrial fibrillation. The prevalence of atrial fibrillation increased markedly after 45 years of age.27 Arrhythmias are an important cardiovascular reason for hospitalization of ACHD and are an increasingly frequent cause of morbidity and mortality.1,28 A population-based study in the Netherlands demonstrated that 31% of cardiovascular hospital admissions in the ACHD population were related to arrhythmias.28 The development of AT has been associated with a greater than 50% increase in the risk of stroke,1 whereas patients with intracardiac shunts and transvenous leads are deemed to be at even higher risk of stroke, estimated at a sixfold to sevenfold increase.29 New-onset AT can contribute to symptoms of palpitations, dizziness, chest pain, and syncope. In addition, it can herald worsening hemodynamic outcome, and the risk associated with AT can be amplified in the presence of the abnormal underlying circulation. For example, in patients with surgically corrected TOF, significant pulmonary regurgitation and left ventricular dysfunction are important risk factors for AT,25,27 whereas in other CHD defects elevated pulmonary artery pressures are an important risk factor for the development of AT.11,22 The development of AT confers a twofold to threefold increased risk of heart failure and has been associated with an increased risk of death from heart failure.1,2 Therefore, the onset of AT should prompt the clinician to look for hemodynamically reversible causes of AT. Arrhythmias, especially AT, are common during pregnancy in women with CHD, with a prevalence of approximately 5% of completed pregnancies, and are associated with an increased risk of adverse fetal complications.30,31 Risk factors for developing arrhythmias during pregnancy are similar to those already described and include a prior history of arrhythmias and extensive atrial surgery (e.g., Fontan, Mustard, or Senning operation).30,31 Bouchardy et al.1 demonstrated in a large ACHD population-based study (n = 38,428) that the presence of AT increases the risk of mortality by approximately 50%. A Dutch population-based study (n = 6933) also showed that AT was associated with all-cause mortality when corrected for age, gender, and severity of underlying CHD (adjusted hazard ratio of 1.8).2 Previous lesion-specific studies demonstrated an increase in mortality in patients with CHD and AT, particularly in Mustard or Senning patients,12 TOF patients,25 and Fontan patients.32 For the overall ACHD population, AT is associated with death owing to heart failure (hazard ratio of 5 : 1),2 whereas AT is also associated with sudden death in Mustard and Senning patients12 and thromboembolic death in Fontan patients.32 The ACHD population consists of different CHD subgroups with different anatomic diagnoses and background risks for death. Risk factors for mortality in the specific subgroup of ACHD with AT include poor functional class, UVH, pulmonary hypertension, and (residual) valvular lesions. ACHD with more than one risk factor have a 5-year mortality rate of approximately 30%.33 The most commonly encountered AT mechanism in ACHD is that of macroreentrant AT, followed by atrial fibrillation.34 Focal AT, AVNRT, and AVRT occur less frequently.35 The term IART is used most commonly to describe the atrial reentrant tachycardias encountered in the patient with CHD. With the exception of Fontan patients, cavotricuspid isthmus (CTI)-dependent AFL remains the most common mechanism of macroreentrant AT in patients with CHD.36–39 Some authors prefer to use the term IART for both AFL and non-CTI-dependent IART.3,40 In patients with CHD, the propensity to IART is facilitated both by natural anatomic and surgically created barriers that result in delayed conduction. A variety of conduction barriers that confine IART circuits (e.g., the right AV valve, vena cavae, crista terminalis, surgical suture lines, patches, baffles, and conduits) have been described in these patients.38 In addition, chronic cyanosis, increased atrial wall stress, and increasing age contribute to the development of abnormal atrial myocardium and fibrosis (Figure 78-2). Patchy islands of scar surrounded by viable myocardium facilitating reentry have been described at the time of intracardiac mapping.41 The most common critical corridor of conduction delay in IART in ACHD is the area between the right lateral atriotomy scar and the inferior caval vein.36,38
Atrial Tachyarrhythmias in Adults With Congenital Heart Disease
Epidemiology
Changing Epidemiology of Congenital Heart Disease
Risk of Atrial Tachyarrhythmias in Congenital Heart Disease
Populations at Risk
Ebstein Anomaly
Complete Transposition of the Great Arteries
Univentricular Heart with Fontan Surgery
Atrial Septal Defect
Tetralogy of Fallot
Clinical Impact
Morbidity
Mortality
Mechanism of Atrial Tachyarrhythmias
Macroreentrant Atrial Tachycardia
Atrial Tachyarrhythmias in Adults With Congenital Heart Disease