CHAPTER 114 Atrial Septal Defect and Cor Triatriatum
ATRIAL SEPTAL DEFECT
Historical Considerations
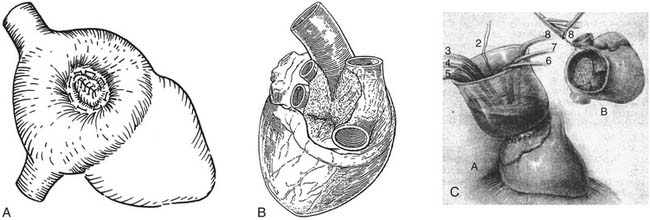
A variety of ingenious surgical corrections of atrial septal defect (ASD) predated the advent of cardiopulmonary bypass. Closed approaches included a blind technique in which a straight needle and suture were passed through both atria, guided by palpation, and the free walls of the left and right atria were drawn together to obstruct the defect.1 Bailey and colleagues described the “atrio-septo-pexy” consisting of a digital invagination of the atrial appendage through the defect with external suture attachment of the atrial tissue to the perimeter of the defect (Fig. 114-1A).2 Tyge Sondergaard devised a pursestring external suture closure of ASDs by a nearly circumferential dissection around the defect in the plane of the interatrial groove and a plication of its edges (see Fig. 114-1B).3
Semiopen techniques prior to cardiopulmonary bypass included the atrial well technique, in which a right atriotomy was formed, controlled by partial atrial clamping. A 15-cm tall, open-ended rubber cone was then attached to the atriotomy to produce an open column of blood in continuity with the beating heart. Working through the “atrial well” by palpation, the defect was closed by direct suture or patch. Regional intermittent heparinization prevented blood clotting in the well (see Fig. 114-1C).4,5
Lewis and Taufic reported the first successful open-heart ASD closure under direct visualization, employing surface cooling and circulatory arrest by inflow occlusion, in 1953.6 Further modification of this technique to include continuous coronary perfusion improved the safety of this and other early intracardiac procedures and was a precursor to modern myocardial protection techniques.7
The modern era of cardiac surgery was heralded by the introduction of the pump oxygenator in 1953, and the earliest application of this technology was for ASD closure.8 Intracardiac repairs by inflow occlusion were not uniformly replaced by extracorporeal circulation techniques until 1960.
Anatomy, Embryology, and Genetics
Formation of the Interatrial Septum
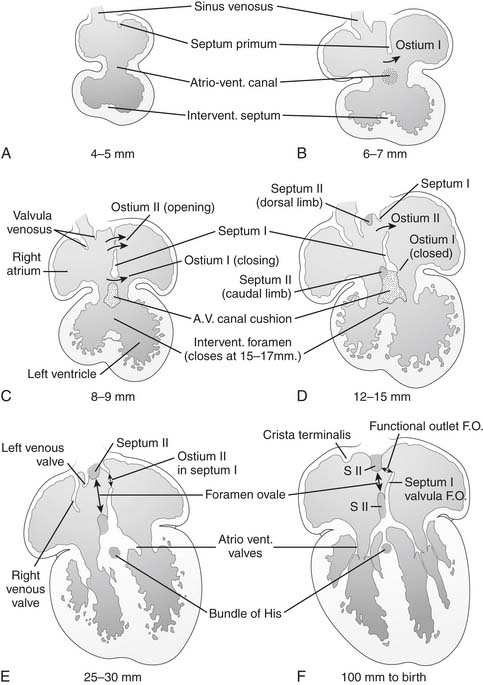
The embryonic common atrium undergoes partitioning by the formation of two parallel, overlapping septa, starting in the 4th week of gestation. The crescentic septum primum, emerging at the superior and posterior aspect of the left atrial heart field,9 begins to septate the atria as the endocardial cushion is forming below to septate the ventricles. The ostium primum, a gap between the septum primum and the endocardial cushion, closes as the ostium secundum forms by a resorption of the superior aspect of the septum primum. By the end of the 6th week of gestation, the septum secundum begins to form parallel to and immediately rightward of the septum primum, obliterating the remaining ostium primum and circumscribing the fossa ovalis. In its final configuration, the atrial septum consists of the two layers, fused except for the overlapping, offset openings of the fossa ovalis and the ostium secundum. The free edge of the ostium secundum forms a flap valve covering the left side of the fossa ovalis, providing free right-to-left flow through the foramen ovale, until postnatal physiology closes the valve (Fig. 114-2). Conditions impairing the competence of the valve, or abnormalities in the formation of its components, lead to a persistent interatrial communication.
Right and left omphalomesenteric and cardinal veins drain into the left and right sinus horns, which together form the sinus venosus segment of the posterior common atrium. In the 4th week of gestation, the common pulmonary vein orifice forms from leftward of bilateral posterior invaginations of the sinus venosus segment into the mesenchyme of the primitive lung buds. An abnormal persistence of the right-sided anlage of the common pulmonary vein might be the embryologic basis for the development of direct connections of pulmonary veins to the right atrium.10
Genetics
Ten percent of ASDs are familial, with 40% to 100% transmission, suggestive of autosomal dominant inheritance.11–13 Penetrance is lower than expected for a single gene defect, and a multifactorial model of inheritance has been proposed.14 Heterozygous mutations in transcription factors expressed in the heart, including NKX2-5,15 TBX5,16 GATA4,17–21 alpha-cardiac actin 1 (ACTC),22 and alpha-myosin heavy chain (MYH6),23 have been reported in kindreds with familial ASD. Defects in genes encoding the components of sarcomeric contractility may affect normal septal development by delaying normal cardiac looping in the forming heart.22
The conduction system forms concurrently with atrial septation, and PR prolongation can accompany ASD, probably in association with abnormalities of the TBX5 transcription factor gene.24 There may be two forms of autosomal dominant ASD, one isolated and one with PR prolongation.25
Patent Foramen Ovale
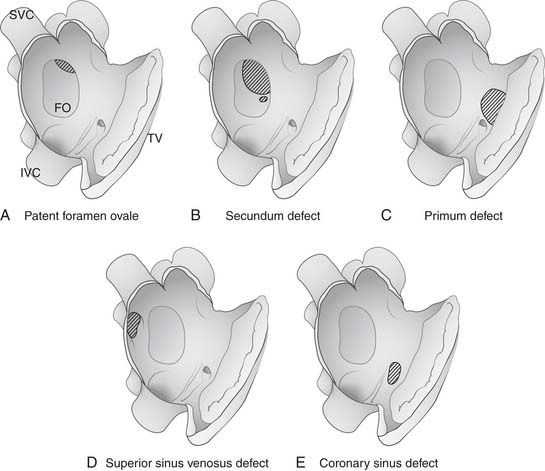
The patent foramen ovale (PFO) denotes a failure of the septum primum and the septum secundum to fuse. A failure of fusion results in either a valve-competent “probe-patent” PFO or valvular incompetence with or without an aneurysm of the septum primum component (Fig. 114-3A). Forces producing an enlarged foramen ovale or a deficient septum primum contribute to incompetence of the valve, a physiologically significant PFO, and trans-septal shunting.
Secundum Atrial Septal Defect
The secundum defect lies within the bounds of the fossa ovalis, and it ranges widely in morphology from the slit-like PFO at the superior aspect of the fossa, to defects involving part or all of the remainder of the fossa, with a single or multiply fenestrated communication. Defects of various specific morphologies classified as secundum ASD can form as a result of an underdevelopment of the septum secundum or a malformation of the septum primum that results in incomplete coverage of the ostium secundum (see Fig. 114-3B).26
Primum Atrial Septal Defect
The primum defect is a persistence of the ostium primum and is most commonly associated with an atrioventricular septal defect (see Fig. 114-3C). This lesion will be discussed in Chapter 116.
Sinus Venosus Defect
The sinus venosus interatrial defect is associated with partial anomalous pulmonary venous return (PAPVR). Most PAPVRs (90%) are right-sided, 7% are left-sided, and 2% are bilateral. The most common subtype occurs at the connection between the right upper pulmonary vein and the superior vena cava (SVC), and it accounts for 74% of PAPVR cases, in usual association with sinus venosus ASD.27 The more common superior variant of sinus venosus defect is an interatrial communication lying posterior and superior to the true atrial septum, and the SVC overrides the defect. The right upper pulmonary veins, usually two or more, drain to the right atrium at the superior cavoatrial junction or enter the SVC directly (see Fig. 114-3D). Rarely, they enter the azygous vein as well. The SVC is commonly enlarged as it enters the right atrium.
Atrial Septal Aneurysm
Redundant atrial septal tissue in the fossa ovalis with a respiratory excursion greater than 10 mm is termed an atrial septal aneurysm (ASA).28 The ASA may occur with or without a PFO and has been implicated in the formation of paradoxical embolus. ASA is present in 2% to 4% of the normal population, and 70% of cases are associated with a PFO.29
Coronary Sinus–Type ASD
The uncommon coronary sinus–type ASD results from a complete or partial unroofing of the coronary sinus along its course through the floor of the left atrium. A communication of the coronary sinus with the left atrium results in an interatrial communication at the level of the coronary sinus ostium, the size of which is determined by the extent of unroofing and the size of the ostium (see Fig. 114-3E). Concomitant cardiac lesions occurring with coronary sinus ASD include ASDs in the fossa ovalis, persistent left SVC, and pulmonary or tricuspid atresia. In a recent study of a series of 25 coronary sinus defects treated at Mayo Clinic, more than half represented a diagnosis missed at previous surgery for other defects.30 The rarity of coronary sinus defects, their tendency to elude echocardiographic diagnosis, and the finding that many elude even intraoperative recognition, underscore the importance of maintaining suspicion for this lesion when the degree of intracardiac shunt observed is inordinate for the known defect elsewhere.
Iatrogenic and Traumatic ASD
Iatrogenic ASDs are found after 87% of catheter-based trans-septal pulmonary vein isolation procedures. A great majority are less than 1 mm in diameter, and 96% resolve spontaneously, requiring no intervention.31 Rarely, traumatic ASD has been described after blunt or penetrating trauma.32
Incidence and Natural History
More than 60% of healthy full-term infants have a PFO.33 With the postnatal fall in pulmonary resistance and rise in left ventricular (LV) end-diastolic pressure, the pressure in the left atrium exceeds that in the right atrium, and the flap valve consisting of septum primum closes the foramen ovale. Fibrous adhesions form in the first year of life to seal the interatrial communication in a majority of cases. Spontaneous closure rates of PFO diagnosed in infancy are high: 87% to 96% for those diagnosed in the first 12 months of life,34,35 and 92% to 99% when diagnosed in the neonate.36,37 The overall incidence of persistent PFO in adulthood, deduced from a study of 965 autopsy specimens, is 27%. A PFO is present in one third of people under 29 years of age, one fourth of those 30 to 79, and one fifth of persons older than 80.38 Secundum ASD occurs in 1 in 1500 live births, accounting for 10% to 15% of congenital heart defects in children and 20% to 40% of defects discovered in adults. Women are affected twice as often as men.39,40 Gestational factors known to predispose to ASD formation include congenital rubella infection, alcohol, hydantoin, valproic acid, and amphetamines.
A significant number of ASDs close spontaneously within the first few years of life, but spontaneous closure after age 3 to 4 is rare.41,42 The likelihood of spontaneous closure is best predicted by the initial diameter of the secundum defect. Recent longitudinal data demonstrate that more than half of defects diagnosed in infancy and measuring 4 to 5 mm close spontaneously. Thirty percent regress in size to less than 3 mm. However, none close spontaneously when the defect measured greater than 10 mm at diagnosis.43 Defects larger than 8 mm at diagnosis usually enlarge. If there is aneurysmal formation, sizes diminish regardless of whether it is greater than 8 mm.44
In contrast to children with an ASD, most patients older than 40 years of age are symptomatic and have evidence of elevated pulmonary vascular resistance. Untreated, the average life expectancy is 40 to 50 years. Seventy-five percent die by age 50, and 90% die by age 60.40,45 Even the asymptomatic adult with ASD has a measurably diminished aerobic exercise capacity, which further declines with advancing age.46 A recent European heart survey of 882 adults with isolated secundum ASD, including 505 unrepaired patients, demonstrated a precipitous rise in the prevalence of right ventricular (RV) dysfunction in unrepaired patients after 45 years of age. The degree of RV volume overload was the best predictor of reduced exercise capacity. A steady rise in the prevalence of pulmonary hypertension was observed in unrepaired patients after age 30. Findings support the suggestion that the magnitude of the intracardiac shunt may increase over time. Hemodynamically small defects tend to remain stable and may not require closure.47
Isolated ASD can predispose the patient to subacute bacterial endocarditis, although the actual incidence of endocarditis in this setting is very low. Atrial septal endocarditis has been described in direct association with native ASD, and to occur by septal involvement of endocarditis that extends from other intracardiac structures.48–52
Associated Features
Isolated ASD in infancy and childhood is seldom symptomatic even for large defects, and symptoms of congestive failure should prompt a careful effort to rule out additional associated abnormalities. Associated lesions found in the study of infants with ASD who died in the first year of life included left-to-right shunting lesions, such as ventricular septal defect (VSD) or patent ductus arteriosus (PDA); right-sided obstructive lesions, such as pulmonary stenosis; and left-sided obstructive lesions, such as aortic stenosis, mitral stenosis, or coarctation of the aorta. Necropsy data show that patients with VSD had an associated ASD in 18% of cases, those with left-sided obstruction had an associated ASD in 29% of cases, and those with right-sided obstructive lesions had ASD in 31% of cases.53 These associations support the hypothesis that some secundum ASDs are acquired, driven by remote lesions that favor persistent atrial-level shunting and atrial dilation, in turn leading to valvar incompetence at the fossa ovalis.
Mitral valve abnormalities have long been recognized as associated with ASD, although their associated incidence is uncommon. Mitral stenosis with pulmonary artery dilation in association with ASD, or Lutembacher’s syndrome, was perhaps a more frequently found association in the era when rheumatic heart disease was more prevalent, although nonrheumatic mitral stenosis is occasionally found in association with ASD.54,55 A cleft anterior mitral leaflet, typically associated with a primum ASD, has also been reported in uncommon association with secundum ASD.56–58 Mitral regurgitation is found in 2.5% to 10% of adults with a large ASD.59 Mitral prolapse may be present in 20% of cases, with a distribution increasing with age.60 Mitral prolapse may be a result of septal distortion by RV volume overload and a secondary effect on mitral valve geometry. As evidence in favor of this hypothesis, mitral prolapse has been shown to reverse after ASD closure.61
Tricuspid regurgitation in association with ASD is usually the result of annular dilation from the enlarged right ventricle, and it also reverses with ASD closure. In a study of 443 patients presenting with ASD or PAPVR, other associated abnormalities included left SVC (5%), mild or moderate pulmonary stenosis (4%), peripheral pulmonary artery stenosis (1%), azygous extension of the inferior vena cava (IVC) (1%), and VSD or PDA (<1%).62 Atrial conduction abnormalities may accompany ASD, in association with a syndrome (notably Holt-Oram), or without apparent syndromic association. P-wave prolongation associated with ASD may predispose the patient to the eventual development of atrial fibrillation.63–65 P-wave duration may be shortened by ASD closure in younger adults, suggesting that the chronicity of atrial stretch is contributory. Closure of ASD in older adults does not affect this electrical pathophysiology and does not shorten preexistent P-wave prolongation. The presence of paroxysmal atrial fibrillation prior to ASD closure results in no shortening of P-wave duration by closure.66,67 Genetic syndromes with associated ASD include Holt-Oram, Rubinstein-Taybi, Okihiro, and Townes-Brocks syndromes.68 Trisomy 21 is associated with ASD as part of a constellation of endocardial cushion defects. Noonan’s syndrome is associated with ASD and pulmonary valve stenosis.69
Hemodynamics and Pathophysiology
Left-to-Right Shunt
In early infancy, when pulmonary resistance is high, left and right ventricular compliances are similar, and net shunting through an ASD is typically slight. As the left ventricle matures postnatally, it becomes thicker and less compliant in diastole than the right and accounts for higher left atrial pressure than right. This drives a left-to-right shunt at the atrial level in the presence of an ASD. With age, the disparity between systemic and pulmonary resistance, and in turn between left and right ventricular compliance, results in increased left-to-right shunting and advancing right ventricular volume loading. Whereas the normal right ventricular diastolic dimension is 0.6 to 1.4 cm/m2, a large left-to-right shunt can result in a right ventricular diastolic dimension as high as 4 cm/m2. Over time, RV volume load results in dilation and hypertrophy, eventually affecting the function of both ventricles. Atrial enlargement may contribute to the late incidence of atrial fibrillation. Right ventricular volume overload is noted to occur, as a rule, when ASDs are greater than 6 mm in diameter.70
Volume-induced hypertrophy of the right ventricle produces a loss of coronary reserve and eventual impairment of RV systolic and diastolic function. LV functional reserve is diminished by adulthood in a majority of patients with ASD. Although LV systolic function may be normal at rest, the left ventricle exhibits a subnormal diastolic dimension, and a loss of functional reserve at exercise. Mechanisms that account for LV dysfunction include septal displacement secondary to RV dilation and hypertrophy, and systolic anterior movement of the mitral valve. In general, the functional loss in the left and right ventricles is normalized 6 months after ASD closure in the child and young adult.71
Pulmonary Vascular Disease
Pulmonary hypertension associated with an isolated ASD is rare in childhood, although 35% to 50% of patients with unrepaired ASD have elevated pulmonary resistance by age 40. The development of pulmonary vascular disease is not uniformly related to age or degree of shunting across the ASD. In contrast, patients with VSD predictably develop pulmonary hypertension earlier and more severely, subjected to similar left-to-right shunting and elevated pulmonary blood flow. An explanation remains lacking for why shunts of similar volume from ASDs or VSDs, generating similar elevations in pulmonary blood flow, produce different patterns of pulmonary hypertension. In a study of 128 patients with ASD and pulmonary hypertension (all older than 18 years), one third demonstrated an elevation in pulmonary vascular resistance (PVR) before 20 years of age, one third between 20 and 40, and the remainder after 40.72
Pulmonary hypertension can develop at an earlier age in premature infants and in children with Trisomy 21. Histopathologic evidence of increased pre-acinar and intra-acinar arterial muscularity in infants with ASD and pulmonary vascular disease suggests that the pulmonary vasculopathy is the primary disorder in the uncommon population with early pulmonary vascular disease, and the ASD may be acquired as a consequence or may be incidentally associated.73
Clinical Presentation
A great majority of ASDs are asymptomatic, and palpitations, atrial fibrillation, and congestive failure are late sequelae, uncommon before 40 years of age. Occasional dyspnea on extreme exertion is observed even in children. Recurrent respiratory infection in the presence of a large ASD is not uncommon. Chylothorax has been reported as a presenting manifestation of ASD, cured by ASD closure.74
Rarely, ASDs are associated with cyanosis. Bidirectional shunting across the ASD without an elevation in the pulmonary resistance has been demonstrated as a source of cyanosis.75 An alternative anatomic source for cyanosis is a streaming of desaturated IVC blood across the ASD, caused by a persistently enlarged eustachian valve that baffles blood flow into the left atrium.76,77 More ominously, cyanosis can develop in the setting of advanced irreversible pulmonary hypertension.
Additional clinical associations with PFO include stroke, migraine headache, high-altitude pulmonary edema,78 and diver’s decompression disease.
Diagnostics and Examination
The electrocardiogram (ECG) from patients with ASD shows RV hypertrophy, lengthened PR interval, incomplete right bundle branch block, and an RSR pattern in V1.1 Electrocardiographic criteria for RV enlargement are found in more than 50% of young patients with a large ASD. A traditional teaching is that the secundum ASD is associated with right-axis deviation and incomplete right bundle branch block, whereas the primum defect exhibits left-axis deviation with right bundle branch block. A normal preoperative ECG was found in only 6% of a study population of sinus venosus ASD.79 Although some of these electrocardiographic findings are reported as useful in children, they are not sensitive diagnostic features in adults80–83 and are seldom referenced in an era when an echocardiographic diagnosis is sensitive, specific, and readily available.
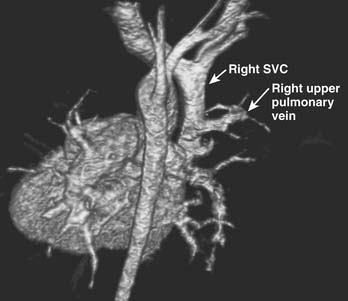
Transthoracic echocardiography with bubble contrast is accepted as the most accurate modality of ASD diagnosis in children, capable of detecting smaller intracardiac shunts than noncontrast echocardiography alone.84 Digital compression storage may delete microbubbles from the image and may account for poorer sensitivity of digital compared with analog echocardiography for detecting intracardiac shunting by bubble contrast.85 Small defects and defects in obscure locations, such as coronary sinus defects and some sinus venosus defects, may remain difficult to image by echocardiogram. High-resolution computed tomography and cardiac magnetic resonance imaging (MRI) have been used to image some interatrial defects that have eluded adequate echocardiographic characterization.86 MRI is particularly useful in imaging partial anomalous pulmonary venous structures, which may lie adjacent to the airways and lung, where air interface interferes with the echocardiographic image resolution (Fig. 114-4).87
Management of Atrial Septal Defect and Patent Foramen Ovale
Indications and Contraindications for ASD Closure
The patients benefiting most from ASD closure are those at risk for developing pulmonary hypertension, but once pulmonary hypertension is present, surgical risk increases. This principle is the basis for the recommendation to close all significant ASDs.88 Elective closure of ASD is generally recommended when the ratio of pulmonary to systemic blood flow (Qp:Qs) is 1.5:1 or greater. Ideally, ASD closure should be performed at age 2 to 5 years, before exercise capacity changes, while chest wall compliance is optimal, and before school age. An echocardiographic diagnosis of a significant defect with RV volume overload is common and sufficient indication to close an ASD. Long-term follow-up data after surgical ASD closure show survival equal to that of the normal population when repair is performed early in life, with an age-related diminution in survival when closure is delayed. The 27-year survival for those repaired after 40 years of age is only 40%.89
Moderate pulmonary hypertension with a reactive component is not a contraindication to ASD closure, although pulmonary hypertension may progress in these patients regardless of closure. In cases of severe pulmonary hypertension, fenestrated ASD closure may be successfully performed, depending on the degree of reversibility and the PVR. Guidelines for inoperability are largely based on VSD data. Generally, the PVR must fall below 7 Wood units/m2 with vasodilator therapy at cardiac catheterization for the ASD closure risk to be acceptable.90 Vasodilators used at cardiac catheterization to determine the reversible component of pulmonary hypertension include hyperoxia, inhaled nitric oxide, and isoproterenol.91
PFO Management
Indications for PFO Closure
The aggressive expansion of catheter-based therapies for PFO has resulted in a proliferation of clinical studies and case reports to reexamine indications for PFO closure. Although it is a subject of some continuing controversy, the data are insufficient to support the medical, surgical, or device management of the incidentally diagnosed, hemodynamically insignificant PFO. Adverse events attributed to the presence of a PFO or associated atrial septal aneurysm include embolic stroke or peripheral embolus,92 brain abscess,93 gas embolization in diver’s decompression illness,94 platypnea-orthodeoxia syndrome,95 migraine headache, and exacerbated hypoxia in settings of pulmonary embolus or elevated right-sided heart pressures after an RV infarct. Indications for PFO closure in these settings are also controversial and are discussed later.
PFO and Stroke
The etiology of stroke in the young adult is unknown in 35% to 50% of cases.96,97 Although PFO is thought to be present in 10% to 20% of the general population, it is present in 40% to 70% of patients suffering cryptogenic stroke.98 Case control studies show a convincing association between PFO and cryptogenic stroke in patients less than 55 years of age. A recent meta-analysis of case control studies showed that, although young patients with a stroke had an odds ratio of 3.1 for having a PFO, young patients with cryptogenic stroke had an odds ratio of 6.0 for having a PFO, compared with patients with known stroke cause.99
Causality after 55 years of age is less clear, probably because of various other sources of ischemic stroke that occur concurrently in the older population. Although the association is not as strong as that in the younger population, PFO independently remains associated with cryptogenic stroke in older patients, with an odds ratio of 3.0.100
Contrary to previous work, recent studies suggest no relationship between size of interatrial shunt and risk of recurrent stroke.101
The association of stroke with PFO is strongest when an atrial septal aneurysm is also present. The absolute risk of ischemic stroke from PFO in the general population is very low, and, in patients less than 55 years of age without an associated ASA, the annual risk of recurrent stroke while taking aspirin is only 1% to 2%. Although the presence of untreated PFO predicts a 2.3% risk of recurrent cerebrovascular event by 4 years after the initial event, the presence of ASA with PFO predicts a 15.2% recurrence risk at 4 years.102,103 In a Rochester, MN, study of 588 individuals older than 45 years, followed for 5 years, the hazard ratio for a first stroke associated with the presence of a PFO was 1.46 and with an accompanying ASA was 3.7.104 These findings further indict the pocket-like septal aneurysm itself as the likely anatomic substrate for the formation of in situ thromboembolus.
Although there is a convincing association between cryptogenic stroke and PFO, the presence of PFO as a predictor of a first or a recurrent stroke is less well established. Meissner and coworkers examined the presence or absence of PFO in 585 healthy subjects older than 45 years as a predictor of cerebrovascular accident. In 5-year prospective follow-up, the incidence of transient ischemic attack (TIA), stroke, or cerebrovascular death was the same in the 24% with PFO as in those without.104 Di Tullio and colleagues found, in 1100 patients older than 39 who had previous stroke, a prevalence of PFO of 14.9%.105 During a 6-year follow-up, incidence of stroke was equal in both groups.
Strategies for treatment of PFO after an ischemic event include PFO closure and medical management with aspirin or Coumadin. Various reports support the superiority of either anticoagulation therapy or mechanical closure of PFO for patients who have suffered cryptogenic stroke.106–109
Obstacles remain to a full understanding of the relationship of PFO to stroke, and to determining the optimal treatment strategy. Echocardiographers may be less likely to find a PFO when another cause of stroke is known. Incomplete device closure may muddy the interpretation of recurrent stroke risk. Antiplatelet therapy, routinely applied after device closure, further confuses the distinction between the effects of mechanical and those of medical treatment. Slow enrollment in randomized trials of medical versus device closure after cryptogenic stroke has delivered sponsors to the point of declaring it not feasible to complete a 2-year follow-up trial with endpoints of stroke or death. Consensus has not yet been achieved, and 2007 U.S. Food and Drug Administration (FDA) recommendations reflect the lack of evidence to support one approach over the other. The 2007 FDA panel concluded the following: (1) Randomized controlled trials of PFO closure to prevent recurrent stroke are required. (2) A “proof of principle” trial with pooled data demonstrating that PFO closure does prevent recurrent stroke could allow this question to be answered in a timely fashion, if sponsors are amenable to cooperating and sharing data. “Proof of device” trials demonstrating that an individual device effectively closes a PFO could be done separately. (3) “Off-label” closure should be discouraged. Enrollment in ongoing trials should be encouraged. (4) Patients and physicians should be educated about the lack of evidence of benefit of closure and the need for completion of trials.110
Generally accepted practice guidelines, based on the present data, suggest a treatment strategy as follows111:
PFO and Migraine
The association of PFO with migraine is murky. Pooled data show a low to moderate grade of evidence that PFO is more prevalent in migraine sufferers with aura. It does not seem that PFO is more prevalent in migraine sufferers without aura (odds ratio, 3.21 for migraine with aura; 2.54 for migraine without).112,113 Migraine cure or improvement has been reported in many patients undergoing closure of PFO, most commonly by device. Although as many as 42% to 72% of patients report that symptoms of migraine are cured or improved after closure of PFO, other reports suggest persistence of migraines or rebound migraine headaches after device closure of PFO.114,115 The association of PFO closure and migraine improvement is limited by placebo effect, peri-procedural anticoagulation therapy, short follow-up, and device complications. Closure of PFO cannot firmly be recommended for the treatment of migraine at present.116
PFO and Diver’s Decompression Illness
Bubbles in the left side of the heart are documented by echocardiogram to demonstrate diver’s decompression illness in association with PFO. Underwater pressure and its effects on ventilation elevate the pulmonary resistance, reducing LV preload and increasing right-sided pressures, and favoring right-to-left shunting across the PFO. The overall risk of diver’s decompression illness with PFO is low, with five events per 10,000 divers, but these odds are five times the odds for those without PFO. An oxygen-enriched mixture or PFO closure is reasonable to consider for divers with PFO.117,118
At present, there are insufficient data to firmly support the closure of PFO for diver’s decompression illness, high-altitude pulmonary edema, or systemic oxygen desaturation from right-to-left intracardiac shunting.119
Catheter-Based Treatment
King and Mills reported the first catheter-delivered ASD closure in 1976, using a double-umbrella device and a 23-French delivery catheter. The large-delivery catheter size precluded its use in children.120 The clamshell occlusion device, reported in 1990, could be delivered through an 11-Fr sheath, bringing device closures to the pediatric population.121 Device arm fracture resulted in its redesign, and a variety of other devices appeared and remain in use. Current devices include the CardioSEAL (Nitinol Medical, Boston), the Amplatzer (AGA Medical Corp., Golden Valley, MN),122 the Sideris buttoned occluder (Custom Medical Devices, Amarillo, TX),123 the Das AngelWings device (Microventa Corp., White-Bear Lake, MN),124 the ASDOS device (Osypka Corp., Rheinfelden, Germany),125 the Helex septal occluder (WL Gore & Associates, Inc., Flagstaff, AZ),126 and a transcatheter polyurethane foam patch.127
A majority of ASDs today are closed by catheter-based devices. The Amplatzer and Helex occluders are the only FDA-approved devices at this time. A recent national sampling of community hospital practices found a 58-fold increase in the annual number of devices placed from 2002 to 2004, and surgical closure rates remained constant.128 The success rate and morbidity are nearly equal when comparing device closure with surgical closure. Current published studies comparing the two approaches with anatomically similar defects show a device success rate of 80% to 95.7%, compared with 95% to 100% success for surgical closure, although the success of device closures continues to evolve. Complications requiring treatment (defined to include anemia, arrhythmia requiring minor treatment, post-pericardotomy syndrome, pericardial or pleural effusion, transfusion, fever, wound complication) occur in 0% to 8% of device closures and 23% to 24% of surgical closures, and mean length of hospital stay is 1 day in the device group versus 3.4 days in the surgical group.129,130 Continual advances in the hardware and experience with device closures are improving the success rate of these catheter-based approaches.
Conflicting cost data currently fail to definitively identufy device or surgical closure as the more cost-effective approach.131 The major cost for the surgically closed ASD is intensive care unit cost, whereas the major cost of the device closure is the device itself.131,132 Cost advantage could favor device closure, particularly in light of evolving strategies of early extubation and accelerated postoperative management protocols that reduce length of stay and cost for the surgical approach.
Anatomic determinants that prohibit device closure are the major indications for surgical ASD closure. Defects that make device closure unsuitable include those that have failed an attempted device closure, common atria or those without sufficient septal rim to engage the device, and sinus venosus defects for which device closure would threaten obstruction of pulmonary veins, IVC, or SVC. Anterior-inferior septal deficiency can be prohibitive of device closure, as the device can interfere with the tricuspid valve, the mitral valve, or the coronary sinus. Individual deficient septal rims, while originally constituting a contraindication to device closure, no longer are absolute contraindications but may reduce success rates.133 The largest Amplatzer septal occlusion device presently available in the United States is 38 mm, and defects exceeding this size would require surgical closure. Multiple defects can be closed with multiple devices, but the cost of multiple device closures may exceed the cost of surgery. Determinants of the limitations to device closure are evolving as devices and their delivery systems continue to undergo refinements.134
Reported complications of device closure of ASD include perioperative or late cardiac perforation (aorta or left atrium),135–139 aorta-to-right atrium fistula,140 device malposition or embolization,141–144 residual shunt,145,146 late secondary ASD formation resulting from septal erosion,147 vascular trauma, thrombus formation,148 tricuspid or mitral regurgitation,142 atrial arrhythmias, device-related infectious endocarditis,149–152 recurrent cerebral embolism,153 entanglement of the device in a Chiari network or eustachian valve,154,155 IVC or coronary sinus obstruction,156 pulmonary vein obstruction,157 nickel toxicity,158–160 late acute tamponade,161,162 and sudden death.135,163 Major complications of device closure reportedly occur in 1.5% of cases, and minor complications in 7.9%.108 Reported noncardiac complications include iliac vein dissection, retroperitoneal or groin hematoma, and leg ischemia.163–166
Zero percent to 8% of patients undergoing percutaneous closure of an ASD or a PFO require surgical intervention for device failure or the management of complications.135,163,167 Of complications, device malposition or embolization is the most common, occurring in 0.2% to 3.6% of cases.126,130,149,168 Cardiac perforation or device erosion may occur in 0.1% of patients, almost exclusively occurring in the dome of the left atrium,169 associated with larger devices and deficient aortic rim.170 Mitral or tricuspid regurgitation can result from device entrapment in chordae, from chordal rupture, or from leaflet perforation by the device or the delivery system.
Additional considerations limiting the catheter-based approach include the risk and impracticality of the peripheral delivery system in infants and small children,171 and the potentially deleterious effects of the requisite radiation exposure.172
As the experience with devices matures, their indications and limits are better defined. Careful avoidance of device oversizing, especially in patients with a deficient aortic rim, may prevent erosion or perforation.171 Absent posteroinferior rims may increase risk of dislodgement, and patients with this anatomy might more safely be served by surgery.173
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree