Fig. 8.1
The inner geometry of the right atrium showing the reentrant loop of a typical flutter circuit. Notice that the typical flutter circuit passes between the IVC and the TV passes in a counterclockwise loop around the TV. This mechanism results in the classic P-wave morphology as the circuit proceeds from low RA near the CS to depolarize the atrium, then reenter the IVC/TV isthmus
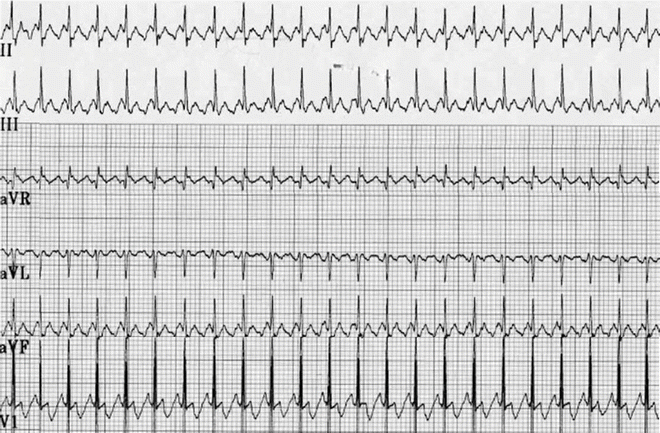
Fig. 8.2
A partial ECG of atrial flutter in an infant. Note the saw tooth appearance with an atrial cycle length of 160 ms and with the 2:1 A:V conduction
This chapter will focus on the diagnosis and management of both atrial flutter in the child and incisional IART in the patient following congenital heart disease surgery.
Background
Atrial flutter comprises less than 1 % of arrhythmias in children with a structurally normal heart; most are infants (Fig. 8.2). In infants, atrial flutter is commonly an isolated event with infrequent recurrence; some authors report a late association with other forms of SVT. On the other hand, only 6–7 % of older children with atrial flutter or IART have a structurally normal heart.
Mechanism of Atrial Flutter
Experimental animal studies and clinical observations have shown that in order for atrial flutter to develop, suitable substrates are required. Prerequisites include:
1.
A corridor or isthmus bounded by structural barriers such as the tricuspid valve, the coronary sinus, the superior or inferior vena cava, or a “scar.”
2.
A functional change in the myocardium resulting in prolonged intra-atrial or inter-atrial conduction and increasing atrial refractoriness.
3.
An eccentric premature depolarization allowing the extrasystole to enter and propagate around the structural barriers.
Functional barriers, such as the crista terminalis with its anisotropic properties, may play an important role in the initiation and maintenance of atrial flutter.
ECG Characteristics
Type I atrial flutter has characteristic inverted “saw tooth” P-waves in leads II, III, and AVF, implying inferior to superior atrial activation typically the reentrant loop consists of a counterclockwise rotation around the tricuspid valve through the cavo-tricuspid isthmus. The anatomic barriers are the tricuspid valve annulus, coronary sinus, inferior and superior vena cavae, and crista terminalis (Fig. 8.1). In infants, these rates are typically very fast, ranging from 350 to 500 bpm (Fig. 8.2). Atrial rates in older children and adults are usually around 300 bpm (range: 240–350 bpm). There is usually variable atrioventricular (AV) conduction yielding a pulse rate of near 150 bpm; in clinical practice, a fixed heart rate of 120–150 bpm strongly suggests the presence of atrial flutter with 2:1 AV block (Fig. 8.3). A second form, atypical atrial flutter, has positive P-waves in leads II, III, and AVF, and usually has slower atrial rates around 200 bpm. The upright P-waves imply superior to inferior atrial activation typically with a clockwise rotation around the tricuspid valve still passing through the cavo-tricuspid isthmus.
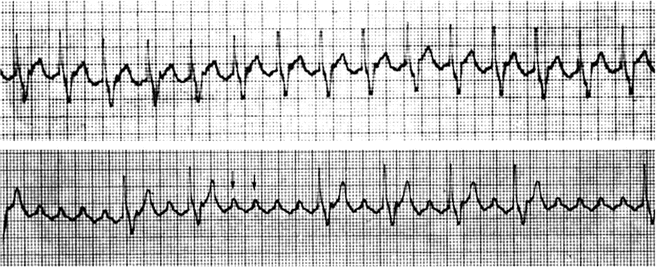

Fig. 8.3
Tracings from a 24-h Holter monitor in a 15-year-old boy with Ebstein’s anomaly of the tricuspid valve. Top tracing: The fixed ventricular rate of 130 bpm strongly suggests atrial flutter with 2:1 conduction, which is confirmed in the lower tracing when greater AV block occurs and the flutter waves become more striking and clear (arrows). Notice the saw tooth appearance
Treatment of Typical Atrial Flutter During an Event
There are several methods of treating this arrhythmia including observation, antiarrhythmic medications, atrial overdrive pacing, and DC cardioversion. For the treatment during an acute arrhythmia, the initial goal of management in the young is termination of the arrhythmia. Rate control is less often employed because of the infrequent recurrence in the child with a normal structural heart, the difficulty in slowing the ventricular rate with cardiac glycosides, beta receptor, or calcium channel blockers (Chap. 22) due to relatively rapid conduction in the young AV node, the desire to avoid anticoagulation in the young, the risk of a negative inotropic effect, especially in the very young, and the success of overdrive pacing or DC cardioversion.
In the newborn and infant, spontaneous conversion is common; however, if the arrhythmia lasts longer than 24 h or if there is clinical deterioration, either cardioversion or overdrive pacing is warranted. Because the atrial flutter is usually composed of a single highly organized reentrant circuit, minimal biphasic energy (0.5–1.0 J/kg or less) delivered through patches placed in the anterior/posterior position is typically all that is necessary.
Transesophageal atrial overdrive pacing (Fig. 8.4) offers the advantage of rapid termination with targeted electrical energy (i.e., to the atria not the entire myocardium). As the atrial flutter rates are often 400 bpm or greater in the newborn and infant, very rapid atrial pacing is required. Transesophageal pacing stimuli are delivered at rates 20–25 % faster than the flutter rate until the flutter circuit is entrained (Chap. 3). When both the orthodromic limb and the antidromic limb of the reentrant circuit are sufficiently engaged by the pacing impulse (wavefront) so as to collide and self-extinguish within the circuit, the pacing is terminated and sinus (or a slower atrial) rhythm will restart. These steps are repeated with faster pacing rates until there is either termination of the flutter or loss of atrial capture. Transesophageal atrial pacing requires higher pacing outputs about 10–15 mA at 4–6 ms pulse duration or greater.
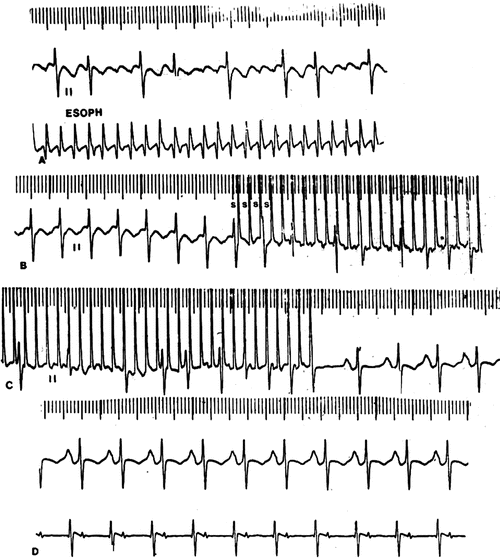

Fig. 8.4
Transesophageal overdrive pacing and conversion of Atrial Flutter (A) in a 12-year-old girl. The pacing cycle length is slightly shorter (B) than the tachycardia cycle length and engages both the orthodromic and antidromic limbs of the reentrant circuit (C) where the impulse traversing both limb collides and self-extinguishes, terminating the flutter followed by emergence of the sinus impulse (D)
Medication for chemical cardioversion may also be considered, and sotalol, ibutilide, and amiodarone have all been shown to be successful in acute conversion ≥60 % of the time when given as an infusion or a bolus in the acute. However, these patients must be carefully monitored as these treatments may be associated with an increased risk of ventricular arrhythmias during their delivery.
To Prevent Further Episodes of Atrial Flutter
Patients with a single episode of atrial flutter, especially if presenting as a neonate, often do not need long-term medical prophylaxis. If deciding to use prophylactic medications for arrhythmia control, beta-blockers, sotalol, propafenone, amiodarone, and dofetilide have shown some promise for long-term control of atrial flutter. For the older patient who has recurrence of atrial flutter and does not respond to pharmacological management, radiofrequency ablation is an option and has been shown in adult subjects to have a greater success (80 % vs. 50 %) than pharmacological management. In patients with repeated episodes of IART, anticoagulation may need to be considered.
Incisional Intra-Atrial Reentrant Tachycardia
Incidence
In contrast to typical atrial flutter, incisional intra-atrial reentrant tachycardia (IART; Fig. 8.5a, b) is an increasingly more prevalent arrhythmia in the young cardiac patient following heart surgery. The vast majority of young patients presenting with IART have heart disease (about 84 %), most of them following surgery. The incidence of incisional IART is related to both the underlying congenital heart defect and the type of surgical repair. In general, the more complex the defect and the surgery (number of incisions and suture lines within the atria), the higher is the incidence. However with the development and widespread success of the arterial switch operation, replacing the atrial switch operation, the incidence of IART in patients with transposition has markedly decreased.
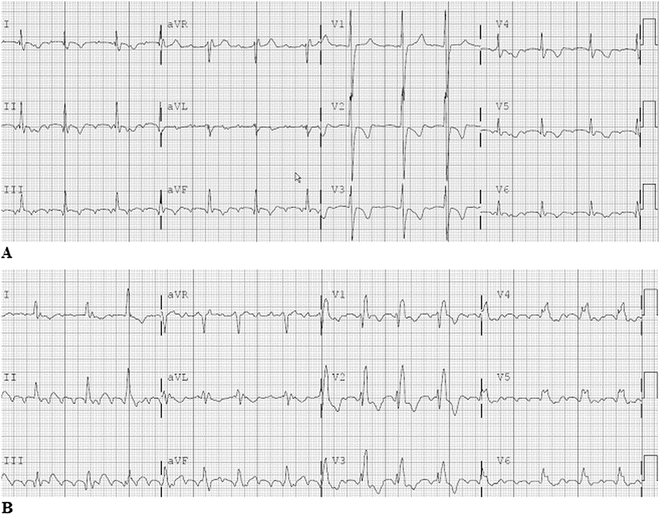

Fig. 8.5
A 12-Lead electrocardiogram in a young adult with L-transposition of the great arteries, Ebstein’s anomaly with tricuspid valve replacement and intra-atrial tachycardia. Note the very small reentry P-waves, especially in the limb and lateral precordial leads. B 12-Lead electrocardiogram in a young adult with repaired Tetralogy of Fallot. This incisional IART was located near the atriotomy incision
A unique and increasingly prevalent group of patients is those who underwent single ventricle palliations (Fontan sequence). The incidence of incisional IART appears to increase with age and to be directly related to the age at operation, the type of operation (most prevalent in the direct atriopulmonary connection), and the presence of sinus node dysfunction. Thus, a substantial number of Fontan patients (16–50 %) may develop incisional IART in their lifetime. Additionally, IART may be associated with an increased mortality, as one study of atrial flutter in the young; the congenital heart disease population had a mortality rate of 5 % in patients with medically controlled IART, and 20 % in medically uncontrolled patients.
ECG Characteristics of Incisional IART
In contrast to typical atrial flutter, incisional IART usually has a slower atrial rate—often less than 300 bpm (cycle length ≥200 ms). Atrial rates of 185–270 bpm (cycle length: 325–220 ms) are seen in Mustard patients and rates of 220–325 bpm (cycle length: 230–470 ms) in Fontan patients. The P-wave morphology is variable, often smaller and fractionated, and at times can be difficult to detect, particularly when evaluating on a few leads, typical with many event monitors. A multiple-lead ECG tracing is often necessary to detect the small P-waves, though a fixed ventricular rate of 100 to 150 bpm in patients following extensive atrial surgery or abnormality should suggest IART with 2:1 conduction to the ventricles. Furthermore, as the atrial rate slows, the possibility of 1:1 AV conduction increases, resulting in faster ventricular rates and an increased risk of hemodynamic compromise.
Etiology
The primary difference between the typical form of atrial flutter and incisional IART is the nature of the barriers around which the reentry impulse circulates. In contrast to typical atrial flutter, incisional IART may involve not only the anatomically fixed structural barriers but also incisions, suture lines, patches, and baffles within the atria, as well as areas of fibrosis. The atria of patients with heart disease are also often subjected to increased pressure and volume loads, resulting in progressive fibrosis and changes in the electrophysiological properties. Clinical intracardiac electrophysiologic studies have confirmed the vulnerability of patients for IART after the Senning, Mustard, and Fontan procedures. This liability is likely due surgically created atrial discontinuities; focal atrial scarring and conduction block associated with suture lines; atrial fibrosis and thickening caused by pericardial inflammation and abnormal wall stress; abnormal atrial size and anatomy; increased atrial refractoriness associated with sinus node dysfunction; and prolonged duration of atrial activation. Many of these abnormalities may even be present before any of their operations. A number of experimental animal studies have reinforced the critical importance of the natural anatomical barriers in typical atrial flutter and found that surgical incisions and suture lines could facilitate IART, especially the crista terminalis, as well as potentially prevent inducibility of IART.
Mustard or Senning Patients
A major vulnerable site for IART in Mustard or Senning patients is near the triangle of Koch (location of AV nodal reentry tachycardia) and the IVC to TV isthmus, both of which are often separated from the systemic venous drainage by the baffle so that some or all of the target triangle is not easily accessible (requiring trans-baffle approach to the target area). Thus, the critical isthmus may either be near the coronary sinus on the systemic venous side or near the tricuspid annulus on the pulmonary venous side or involve both sites. A second common site for IART in this population is the junction between the superior vena cava and systemic venous atrium (Fig. 8.6).
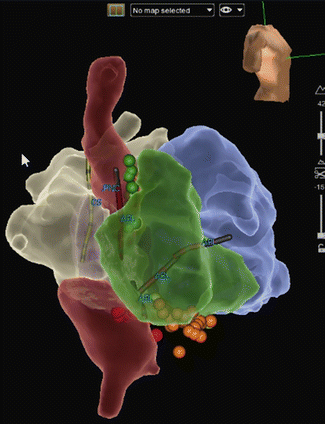

Fig. 8.6
A right lateral, slightly RAO 3-D map (St. Jude, Ensite Velocity) of an adult patient with D-Transposition of the Great Arteries who underwent a Mustard procedure as an infant. The systemic venous pathway (SVC and IVC baffle side) is displayed in red, the pulmonary venous pathway (pulmonary vein baffle side), in white; RV (green) and LV (blue). Two IART circuits were ablated, one on the pulmonary venous side across the TV/IVC isthmus (red and orange lesions markers), and a second in the SVC to atriotomy area (green lesion markers)
Fontan Patients
In post Fontan patients with IART, intra-atrial mapping studies have demonstrated several conduction corridors or isthmuses between the atriotomy incision and the crista terminalis, around the atrial septal defect patches or between the inferior vena cava and tricuspid valve (Fig. 8.7, Table 8.1).
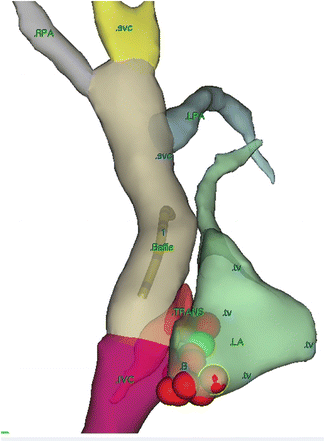

Fig. 8.7
An RAO view using 3-D mapping of a lateral tunnel Fontan with IART near the TV/IVC isthmus with successful ablation after a trans-baffle procedure. The SVC IVC and baffle are seen connecting to the right and left pulmonary arteries
Table 8.1
Types of congenital heart disease and their associated surgeries and common locations of IART
Congenital heart disease | Common locations for IART |
|---|---|
D-TGA with Mustard or Senning | IVC and tricuspid valve isthmus—often on the pulmonary venous side SVC and systemic venous atrium junction |
Fontan | Isthmuses between atriotomy incision and the crista terminalis Around the atrial septal defect patches IVC and tricuspid valve isthmus Combination of all the above |
ASD (and procedures with ASD repair) | IVC and tricuspid valve isthmus Atriotomy site ASD patch |
Treatment of Incisional IART or IART in a Patient with Congenital Heart Disease
Observation
Treatment of IART is dependent on several factors including: associated heart disease, frequency and duration of episodes, associated hemodynamic compromise, patient/parental preference, and failure of previous management strategies. For patients who have had a single, well tolerated, self-limited episode of incisional IART, observation as the initial strategy after cardioversion or atrial overdrive pacing is an option. These patients are advised on the importance of early physician notification of recurrence as sustained tachycardia increases the risk of both hemodynamic compromise and thrombus formation (anticoagulation discussed below).
Medical Management
As incisional IART tends to be a progressive disease, most patients will require antiarrhythmic therapy at some point (Chap. 22) and anticoagulation will need to be considered. Unfortunately, this arrhythmia can be difficult to control, and, not uncommonly, as many as 2–3 antiarrhythmic drugs per patient may yield only suboptimal results. In patients who have infrequent episodes of IART or have had significant hemodynamic compromise due to rapid AV conduction, rate control should be considered in addition to anti-platelet or anticoagulation therapy. Beta-blocker and calcium channel blockers (diltiazem) are first-line agents. Beta-blockers may suppress sinus node function in an at-risk population, as well as possibly depressing ventricular function. Calcium channel blockers can exert a negative inotropic effect, but usually are well tolerated.
For patients with frequent or poorly tolerated episodes of IART, both rate and rhythm control is desirable. The mainstays of rhythm control agents include sodium channel blockers and potassium channel blockers as individual agents or in combination. In part, the antiarrhythmic mechanism of action of sodium channel blockers is to slow myocardial conduction velocity. While this can help in preventing reentrant atrial arrhythmias, it can also slow an IART, converting what once was a hemodynamically well-tolerated rapid atrial tachycardia with 2:1 AV conduction to a slower reentrant tachycardia with 1:1 AV conduction and a paradoxically faster ventricular rate. If a sodium channel blocker is used, consideration should be given to the addition of a rate control agent to protect against this adverse effect.
Potassium channel blockers, sotalol and amiodarone, and more recently dofetilide, which prolong the myocardial effective refractory period, are valuable agents in preventing IART. Sotalol and amiodarone also have the beneficial effect of slowing AV conduction. Nevertheless, the side effect profile of these mediations should be considered as dofetilide and sotalol have a higher risk of proarrhythmia while amiodarone has a greater risk of systemic adverse reactions (Chap. 22).
Anticoagulation
While the risk of thrombus formation is unknown, prophylactic anti-platelet or warfarin therapy is recommended for those with frequent or incessant IART, and aspirin should be considered in many patients with IART, even with only infrequent events. For patients with frequent and/or poorly tolerated episodes of IART anticoagulation therapy should also be considered for at least 3–6 months before considering weaning from the anticoagulation if arrhythmia suppression is obtained. In those with an acute episode of IART, the risk of thrombus formation increases with tachycardia duration. Therefore, cardioversion should be considered within the first 24 h if not anticoagulated. An echocardiogram, often transesophageal, is useful to exclude a preexisting thrombus before cardioversion. If one is seen, warfarin therapy should be instituted for at least 3 weeks prior to cardioversion if the clinical state permits.
Electrophysiology Study
Goals for electrophysiologic evaluation include:
1.




Evaluation of possible arrhythmia-related symptoms (palpitations, tachycardia, and syncope) in patients with repaired or palliated congenital heart disease.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


