Atrial Fibrillation
Eric N. Prystowsky
Amos Katz
Overview
Atrial fibrillation (AF) is the most common sustained arrhythmia affecting humans. Pathology studies have found loss of atrial myocardium with fibrosis and fatty infiltration, but many similar changes can occur as a result of aging alone. Maintenance of AF may depend on reentry, with multiple wavelets occurring simultaneously, continuous triggers, or both. The initiation of AF in many patients may be caused by rapidly firing foci, typically in the pulmonary vein(s). Although the atrial rate is rapid, usually greater than 300 beats per minute, the ventricular response depends on atrioventricular (AV) node conduction properties and the level of autonomic tone. AV node conduction is facilitated by sympathetic tone and inhibited by parasympathetic tone. A variety of medical conditions are associated with AF, most frequently hypertension, coronary artery disease, and valvular heart disease. Many patients have idiopathic, or lone, AF. There are three major tenets of therapy: (a) restoration and maintenance of sinus rhythm, (b) ventricular rate control, and (c) prevention of thromboembolism. One or more of these may be indicated in a particular patient. Several antiarrhythmic agents are effective for restoring and maintaining sinus rhythm, and selection of a particular drug depends on many factors, including the presence and type of underlying heart disease, concomitant illnesses, and renal or hepatic dysfunction. Radiofrequency catheter ablation is being used more frequently to cure AF in patients failing drug therapy. β-Adrenergic blockers and calcium channel blockers are more effective than digoxin in controlling ventricular response, although digoxin is the first-line treatment for patients who have congestive heart failure. In patients who have Wolff-Parkinson-White syndrome (WPW), intravenous procainamide or ibutilide is the preferred therapy for blocking conduction over the accessory pathway during AF, and digoxin, adenosine, β-adrenergic blockers, and calcium channel blockers are contraindicated. In patients at high risk for thromboembolism, anticoagulation therapy using warfarin is recommended, aiming for an international normalized ratio (INR) of 2.0 to 3.0. Anticoagulation is also recommended for patients who are undergoing pharmacologic or electrical cardioversion if AF has been present for at least 48 hours.
Historical Perspective
Atrial fibrillation is the most common sustained arrhythmia affecting humans. Interest in it has waxed and waned for decades, and recently it has taken a back seat to research into arrhythmias such as AV reentry (WPW), AV node reentry, sustained ventricular tachycardia, and ventricular fibrillation. However, there has been a resurgence in interest in the mechanisms and treatment of patients with atrial fibrillation, especially in the potential for curing this arrhythmia using intracardiac
catheter ablation techniques. The following is a brief summary of significant individuals who have made important observations concerning atrial fibrillation.
catheter ablation techniques. The following is a brief summary of significant individuals who have made important observations concerning atrial fibrillation.
TABLE 63.1 Etiology of Atrial Fibrillation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Evaluation of the peripheral pulse has fascinated physicians for centuries. In approximately 1187, Moses Maimonides presented aphorisms pertaining to the pulse. He described an irregular pulse that was regularly irregular and one that becomes completely irregular (1). He was at least partially correct when he mentioned, with regard to the origin of the regularity, “all types of pulses which are irregular in more than one beat are a direct result of an abnormal constitution of the heart which is also irregular or due to an affliction arising in the stomach or in one’s strength”. Before the electrocardiographic confirmation of atrial fibrillation, many observant physicians commented on grossly irregular pulses that, in at least some cases, were likely atrial fibrillation. William Stokes (2), in his classic text on heart disease, stated, “It has happened often to me to find the action of the heart, which, for many months together, had been in the highest degree irregular, suddenly restored to a condition in which the rhythm and sounds were perfectly natural.” In 1904, Wenckebach (3) published a monograph on cardiac arrhythmias. He described cases of excessive irregularity, or “delirium cordis.” One such example is accompanied by a pulse tracing that demonstrates a very irregular rhythm, likely atrial fibrillation.
Cushny (4), Mackenzie (5), Rothberger and Winterberg (6), and especially Lewis (7) made significant early observations concerning atrial fibrillation. The magnum opus of Sir Thomas Lewis is a must-read for any student of atrial fibrillation. The pioneering work of Einthoven (8) enabled clinical investigators to record the electrocardiographic representation of the clinical observations of atrial fibrillation.
Epidemiology
AF has a profound effect on morbidity and mortality among hundreds of thousands of patients and on health care costs in the United States (9). One report analyzed 3,806,000 patient hospital discharges in 1990 from 678 hospitals to determine the frequency with which arrhythmia was the principal diagnosis (10). Approximately 1.5% of all hospital discharges listed arrhythmia as a principal diagnosis, and AF accounted for nearly 35% of the arrhythmias noted. The epidemiology of AF has changed substantially since the early part of the twentieth century primarily because of the dramatic decrease in rheumatic fever and the longer life span of the population (Table 63.1) (11,12,13,14,15,16). The patient populations evaluated in Table 63.1 differ substantially. For example, two studies included patients admitted through the emergency department (13,14) and one evaluated only outpatients (15). Nonetheless, it is obvious that rheumatic heart disease plays a relatively minor role in the current etiology of atrial fibrillation in the Western world, whereas hypertension and, to a lesser extent, coronary artery disease currently are major etiologic factors in AF. The incidence of lone AF is quite variable and is influenced by the intensity of the diagnostic workup, the definition of lone atrial fibrillation used, and the patient population studied. Kannel and associates (12) reported a 31% incidence of lone AF, but some of their patients had obvious heart disease identified by an enlarged heart on chest radiography. Thus, these data are problematic. In contrast, Prystowsky and colleagues (15) studied only patients who had no known etiologic factors for AF and normal ventricular function identified by echocardiography. However, in their study, only outpatients were evaluated. It is clear that the prevalence and types of etiologic factors for AF depend on the setting in which AF is evaluated, including the country of origin.
Changes in etiology of atrial fibrillation may affect its course, especially the evolution of paroxysmal atrial fibrillation into established atrial fibrillation. In a study involving 1,212 patients from Denmark, three time periods, 1940 through 1948, 1949 through 1957, and 1958 through 1967, were evaluated (8). Atherosclerotic heart disease was present in 23%, 37%, and 38% of patients, respectively; hypertensive heart disease was present in 8%, 7%, and 10% of patients, respectively; and rheumatic heart disease occurred in 27%, 17%, and 15% of patients, respectively. During follow-up, the transition rate from paroxysmal to established or permanent atrial fibrillation varied according to the etiology of the disease and was 27% among patients with atherosclerotic heart disease, 40% among patients with hypertensive heart disease, and 66% among patients with rheumatic heart disease. Few data are given on the use of antiarrhythmic drugs to prevent transition to established atrial fibrillation. However, given the dates of the study, only quinidine would have been used with any frequency. These data suggest that the transition from paroxysmal to established atrial fibrillation is not inevitable, especially among patients without rheumatic heart disease. It is quite possible that antiarrhythmic therapy may further decrease the number of patients with established atrial fibrillation. This issue is discussed in more detail in the section entitled Therapy of Atrial Fibrillation.
Effect of Age and Gender
The prevalence of AF increases with age and is 0.5% for patients aged 50 to 59 years and 8.8% for those aged 80 to
89 years (17). Men are affected slightly more often than women. In the Framingham data, excluding individuals with rheumatic heart disease, the 2-year incidence of development of AF was 0.04% and 0.00% for men and women, respectively, aged 30 to 39 years, and 4.6% and 3.6%, respectively, for men and women aged 80 to 89 years (18). Thus, the number of patients who have AF will rise pari passu with the aging of the population. In the first two decades of life, AF is relatively rare (9). When it is found in patients in this age group, it is usually associated with heart disease or the presence of an accessory pathway.
89 years (17). Men are affected slightly more often than women. In the Framingham data, excluding individuals with rheumatic heart disease, the 2-year incidence of development of AF was 0.04% and 0.00% for men and women, respectively, aged 30 to 39 years, and 4.6% and 3.6%, respectively, for men and women aged 80 to 89 years (18). Thus, the number of patients who have AF will rise pari passu with the aging of the population. In the first two decades of life, AF is relatively rare (9). When it is found in patients in this age group, it is usually associated with heart disease or the presence of an accessory pathway.
Pathology
Changes Associated with Aging
The incidence of atrial fibrillation increases with age, and the condition is especially prevalent among individuals aged 60 years or older. Thus, it is important, in attempts to identify the pathologic changes associated with atrial fibrillation, to consider changes that are associated with the aging process in and of itself. Macroscopic and microscopic alterations in atrial tissue begin in the first year of life (19). By the fourth and fifth decades, small fat spots appear in the right atrium in the region of the AV node and septum. There is accentuation of the thickening of the plaques in later decades. In the left atrium, the endocardial thickening is diffuse. With aging, increased thickening occurs, especially at the mitral valve annulus. Calcification and fatty infiltration may be present in the annulus. Histologic examination reveals an endothelial lining, beneath which is a fibroelastic core. With aging, there is focal or diffuse proliferation of smooth muscle cells, elastic fibers, or both and, in some cases, collagen fibers. This process has been termed endocardial hypertrophy (19). In the fourth decade, hypertrophic and sclerotic changes occur in previously uninvolved portions of the right atrium, and in the fifth decade, atrophy of smooth muscle layers can be seen. Increased sclerotic changes occur in the sixth decade. By the fourth decade, the entire left atrium appears to be affected by hypertrophy and sclerosis, and collagen replacement is frequent. The changes associated with aging result in eventual loss of myocardial fibers and an increase in fatty metamorphosis and connective tissue in the sinus node, the AV node, and the atrial approaches to these structures (19).
Pathology of the Atrium in Atrial Fibrillation
One study analyzed pathologic changes in the atria in 145 patients with AF and control patients (19). Etiologies were diverse and included conditions such as rheumatic fever, hypertension, hyperthyroidism, and coronary artery disease. The author speculated that no specific histologic syndrome is associated with AF. In another study, lesions of the sinus node were evaluated in the hearts of 65 patients (20). The anatomy of the sinus node was compared with clinical data in a blinded manner. The sinus node was obviously damaged in 15 patients, and an established arrhythmia, usually AF, was seen in 14 patients. The sinus node was normal in 49 patients, and AF had been present in 5 patients. The common association in this study between sinus nodal damage and a clinical history of AF supports the idea that sick sinus syndrome is a panatrial disorder in many patients.
One of the most important pathologic studies of the atria of patients with AF was done by Davies and Pomerance (21). These authors analyzed the hearts of 100 patients with AF and grouped them into patients who had AF for less than 2 weeks before death and those who had AF for more than 1 month before death. Among patients who had the longer-term AF, cor pulmonale, rheumatic heart disease, and ischemic heart disease were the most frequently associated clinical conditions. In nearly 75% of cases of chronic AF, sinus node muscle loss, internodal tract muscle loss, and atrial dilation of some degree were present. Notably, left atrial appendage thrombosis was identified in 46 patients with long-term AF and cerebral infarction was identified in 19. However, only 3 of 19 patients with short-term AF had left atrial thrombus and only 1 patient had cerebral infarction. These authors offered an important hypothesis concerning the origin of pathologic changes in the atria. They stated that “while it is conventional to regard the fibrotic changes in the node and atria as the cause of atrial fibrillation, it is also possible that they result from the arrhythmia and consequent disordered function of the chamber” (13). This prescient observation supports the current belief that AF begets AF, therefore making it possible to retard or even prevent the development of permanent AF in some patients by maintaining sinus rhythm, which may beget sinus rhythm.
Pathophysiology
Electrophysiologic Mechanism of Atrial Fibrillation
Arrhythmias require an initiating event, for example, a premature atrial or ventricular complex, and a sustaining substrate, for example, one or more reentrant circuits. The initiating event and sustaining substrate may be all due to automaticity such as a rapidly firing atrial focus. In AF, both automaticity and reentry appear to play a role.
Automatic Focus Theory of Atrial Fibrillation
The ectopic focus theory of AF was championed by Scherf and coworkers (24,25). Topical application of aconitine to the appendix of the right or left atrium yielded arrhythmias that were similar in appearance to AF. Clamping off the area of application from the rest of the atrium allowed the arrhythmia to continue in the area that was clamped off, but not in the rest of the atria. The experiments with aconitine suggested that AF depended on a single focus.
The ectopic focus theory for AF was essentially smothered by the overwhelming weight of observations indicating that reentry was the mechanism of AF. However, observations made during intracardiac radiofrequency catheter ablation of AF have rekindled interest in the ectopic focus theory (26,27,28). These authors (26,27) were able to terminate AF with discrete applications of radiofrequency energy, primarily in the area of the pulmonary veins (PVs) in the left atrium, although other atrial sites including the superior vena cava, ligament of Marshall, crista terminalis, coronary sinus, and left posterior free wall, have also been identified (28,29,30,31,32). The PVs are most frequently the origin of these rapid atrial foci, and studies have confirmed that cardiac muscle extends onto the PVs in humans (33), and PVs have automaticity in an experimental model (34).
In patients with AF, PVs have shorter refractory periods than in control patients, and refractory periods are also shorter in the left atrium outside of the PVs (35). Decremental conduction in PVs can occur, and the heterogeneity of conduction could promote reentry in the PV or PV–left atrial juncture (36). The rapid local left atrial activation cannot be conducted in an
organized way to the right atrium. Experiments in sheep hearts demonstrated a dominant fibrillation frequency in the left atrium, and this decreased with activation to the right atrium (37). This fibrillatory conduction likely explains the chaotic atrial rhythm typically noted in the electrocardiogram (ECG). However, it is often possible to observe in ECG lead V1 well-defined P waves with a short cycle length that is irregular.
organized way to the right atrium. Experiments in sheep hearts demonstrated a dominant fibrillation frequency in the left atrium, and this decreased with activation to the right atrium (37). This fibrillatory conduction likely explains the chaotic atrial rhythm typically noted in the electrocardiogram (ECG). However, it is often possible to observe in ECG lead V1 well-defined P waves with a short cycle length that is irregular.
Multiple-Wavelet Reentry Theory of Atrial Fibrillation
A key development in our understanding of the mechanism of AF was the multiple wavelet hypothesis proposed by Moe and coauthors (38,39). They noted, “the grossly irregular wave front becomes fractionated as it divides about islets or strands of refractory tissue, and each of the daughter wavelets may now be considered as independent offspring. Such a wavelet may accelerate or decelerate as it encounters tissue in a more or less advanced state of recovery” (38). Thus, the larger the number of wavelets that present, the more likely it is that the arrhythmia will sustain. The number of wavelets depends on the atrial mass, the refractory period, and the conduction velocity of various areas of the atria. In essence, a large atrial mass with short refractory periods and conduction delay would yield increased wavelets and would present the most favorable situation for AF to be sustained.
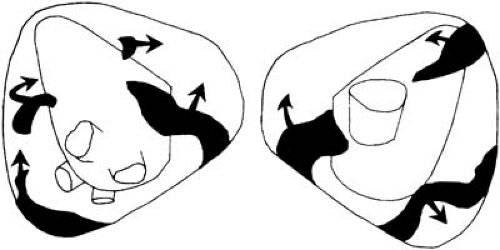
Experimental validation of the multiple-wavelet hypothesis was demonstrated by Allessie and colleagues (40). They analyzed the excitation of canine atria during induced AF with two egg-shaped multiple electrodes inserted into the cavities of the atria. Each electrode contained 480 recording electrodes, with an interelectrode distance of 3 mm. This enabled the first highly detailed activation sequencing of AF. Figure 63.1 demonstrates an arbitrary moment during AF and reveals seven wavelets (40). These reentrant wavelets were not stable, and over the course of time, various pathways of reentry could be demonstrated. When only three wavelets existed, there was a high chance that all would cease to exist, but when six wavelets were present, spontaneous termination of AF did not occur.
The mechanism of initiation of AF is not certain in most cases and likely is multifactorial. For example, it may occur secondarily from another arrhythmia, so called tachycardia-induced tachycardia (41) (Fig. 63.2). In such circumstances, the initial tachycardia is the actual mechanism for induction of AF, and might be related to the tachycardia cycle length, intrinsic atrial vulnerability, contraction-excitation feedback, or a combination of these factors (41). If reentry is assumed to be the mechanism of AF, initiation would require an area of conduction block and a wavelength of activation that is short enough to allow the reentrant circuit in the myocardium. The normal aging process results in anatomic changes likely to yield inhomogeneity in conduction that may create the milieu necessary for the development of reentry (42,43). These changes are likely magnified by the presence of certain disease processes, for example, those of coronary artery disease. Clearly autonomic tone influences AF initiation, especially enhanced vagal activity. The roles of increased atrial pressure and volume and activation of stretch-induced ionic channels require further study.
Tachycardia-Induced Atrial Cardiomyopathy
It has been recognized for several decades that a persistently fast ventricular rate secondary to supraventricular tachycardia can lead to ventricular cardiomyopathy, which is reversible if recognized in time. In fact, this is not an uncommon phenomenon in patients who present with AF and dilated cardiomyopathy, especially in those patients without palpitations. A tachycardia-induced atrial cardiomyopathy can also occur (44). The effect of persistent AF on changes in atrial size was investigated in 15 patients who showed no evidence of significant structural or functional cardiac abnormalities other than AF (45). During an average follow-up period of 20.6 months, mean left atrial volume increased from 45.2 to 64.1 cm3 and right atrial volume significantly increased from 49.2 to 66.2 cm3. In contrast, after cardioversion of AF at 6 months’ follow-up, in 28 patients in whom sinus rhythm was maintained the left atrial volume decreased from 72.6 ± 15.1 to 58.5 ± 13.8 cm3 and right atrial volume decreased from 68.7 ± 14.6 to 58. ± 11.6 cm3 (p < .05), but atrial size did not change in patients in whom AF recurred (46).
Evidence for a tachycardia-induced atrial cardiomyopathy is supported further by echocardiographic observations before and after cardioversion (47,48




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree