Asthma: Clinical Presentation and Management
ASTHMA—A HETEROGENEOUS DISEASE
Asthma is a chronic inflammatory disorder of the airways characterized by marked variability in airflow obstruction that is often reversible, either spontaneously or with treatment.1 This inflammation presents clinically in susceptible patients with recurrent symptoms of wheezing, chest tightness, cough, and, occasionally, dyspnea and contributes to the heightened airway hyperresponsiveness to specific and nonspecific stimuli; a pathognomonic feature of asthma. Increased airway hyperresponsiveness manifests in patients as intolerance to smoke, dust, air pollution, and strong odors, where exposure to such agents in healthy individuals does not induce such symptoms. Asthma is not a single disease entity with a unique pathogenesis, but rather recognized to be a clinical syndrome and heterogeneous disease;2 that is, asthma comprises multiple endotypes that manifest common symptoms, but have distinct and probably different pathophysiologic and etiologic mechanisms with an interplay between genetic and environmental factors. This phenotypic heterogeneity in the expression of asthma is multidimensional and includes variability in pathologic, clinical, and physiologic parameters among different patients.3
RISK FACTORS FOR ASTHMA
Several risk factors for asthma are considered below.
 ATOPY AND ALLERGENS
ATOPY AND ALLERGENS
The most important factor predisposing to asthma is atopy (Table 46-1). Asthma has been classified as atopic (extrinsic) or nonatopic (intrinsic) depending on the suspected role of allergens as etiologic factors. Atopic asthma involves an exaggerated immune response characterized by immunoglobulin E (Ig-E) activation and mast cell degradation. Atopy can be clinically elicited with a positive skin prick test or specific antibodies to IgE in serum against common aeroallergens such as house dust mite, grass and tree pollens, cat and dog fur, rodents (in laboratory workers), and cockroaches (in inner city populations). House dust mite is recognized as a significant cause of asthma throughout the developed world, although the relative importance of different indoor allergens may vary among populations. Patients with atopic asthma commonly suffer from other atopic diseases, including allergic rhinitis that may be seasonal (hayfever), and may be found in over 80% of asthmatic patients; allergic conjunctivitis; and atopic dermatitis (eczema). Nonatopic asthmatic patients (approximately 10%) have a negative skin prick test, normal serum IgE concentrations, and usually show later onset of disease (adult-onset asthma). In this group, their asthma is more severe, persistent, there is more sensitivity to aspirin and commonly they have concomitant nasal polyps. This classification, although appropriate from a pathologic perspective, does not readily help clinicians as it does not aid in establishing an etiologic diagnosis nor does it help in defining treatment strategies.4 There is a high prevalence of atopy among nonasthmatics and a large percentage of skin prick sensitive persons report no allergic symptoms. Around 50% of asthma can be attributed to atopy in the developed world and the prevalence of atopy among asthmatics is mainly determined by the general prevalence of atopy in the population.5,6 In addition, the immunopathology in bronchial biopsies and sputum in patients with nonatopic asthma appear to be identical to that found in atopic asthmatic patients. Therefore, the finding that an asthmatic is atopic does not imply that the disease is allergic in nature or, that atopy is causing asthma. Moreover, respiratory tract viruses have emerged as the most frequent triggers for exacerbations in both children and adults and may play a more prominent role than allergens as triggers of acute exacerbations in most patients.7 House dust mites are the most common indoor allergen, where particles excreted from the digestive tract contain the principal allergen Dermatophagoides pteronyssinus. Other main sources of inhaled indoor allergen are cat and dog fur, and cockroaches (Table 46-1. Although asthmatic symptoms often improve when the allergen is removed, rigorous allergen avoidance has not shown any evidence for a reduced risk of developing asthma.
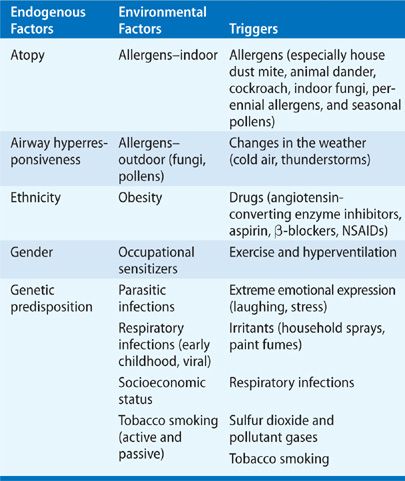
Although allergens are often triggers of acute exacerbations of asthma, allergens themselves may induce subclinical airway inflammation that may lead to enhanced airway responsiveness and greater susceptibility to the provocative effects of other triggers such as respiratory viral infections and exercise. In this regard, it is important to understand the distinction between triggers and etiologic risk factors. A trigger is any agent capable of inducing or exacerbating asthma and whereas triggers may lead to symptoms, they do so only in susceptible persons who already possess the underlying asthmatic diathesis.
 VIRAL INFECTIONS
VIRAL INFECTIONS
Acute upper respiratory tract viral infections are the commonest triggers of exacerbations of asthma and most are due to rhinovirus infections. Viral infections not only give symptoms of the common cold and cause acute inflammatory rhinitis, but may also play a role in asthma development and potentially, airway remodeling through increasing inflammation in the lower airways.8 Asthma is recognized to be more common in children who have had croup or lower respiratory tract infections in early life, although viral infections in the absence of atopy do not appear to be risk factors for the development of asthma.9 Other viruses commonly implicated in acute exacerbations of asthma are respiratory syncytial virus, influenza virus, and parainfluenza virus. Bacterial infection with species of Mycoplasma and Chlamydia are also associated with exacerbations of asthma, whereas other bacterial infections are not.
 OCCUPATIONAL EXPOSURE
OCCUPATIONAL EXPOSURE
Occupational asthma accounts for approximately 5% of all adult cases of asthma, and the disease can often be classified according to its etiology. In these circumstances, not only is the specific agent that triggers the symptoms known, but the same agent is usually the underlying cause of asthma.
 EXERCISE-INDUCED ASTHMA
EXERCISE-INDUCED ASTHMA
Many asthma patients have worsening of symptoms on or after physical exercise and another category of asthma is exercise-induced, where exercise per se is not the cause of, but rather one of many nonimmunologic triggers that produce symptoms in patients who already have the disease. In this condition, the trigger is thought to be the drying of the airway mucosa as a result of hyperventilation that leads to osmotically induced mast cell mediator release and bronchospasm.
 OBESITY
OBESITY
Obesity is a major risk factor for asthma where abdominal obesity (waist circumference) and general obesity (BMI) both show a strong correlation with the risk of new-onset asthma.10
 DRUGS
DRUGS
Drugs that may worsen asthma control include β-blockers, occasionally angiotensin-converting enzyme (ACE) inhibitors, aspirin, and nonsteroidal anti-inflammatory drugs (NSAIDs).
CLINICAL PRESENTATION AND DIAGNOSIS
Asthma is a clinical diagnosis made on the basis of a medical history of typical symptoms, consideration to provocative factors, and supported with objective confirmation of variable airflow obstruction. As the disease is heterogeneous in its presentation and severity, the clinical features of asthma show great variability both between individual asthmatics, and also within the same patient over time. It is also important to recognize that asthma is often associated with different comorbidities including allergic rhinitis, atopic dermatitis, rhinosinusitis, gastroesophageal reflux disease, diabetes, depression, obesity, all of which may affect the clinical expression and severity of the disease.11 The following clinical features and laboratory assessments are important in the consideration of the diagnosis of asthma.
 MEDICAL HISTORY
MEDICAL HISTORY
The typical symptoms of asthma are paroxysmal wheezing, cough, breathlessness, and chest tightness, which may temporally be related to exposure to triggers or exercise. Cough may be productive of clear or yellow/green discolored sputum, where the latter may be tenacious and difficult to expectorate and reflect the underlying airway inflammation rather than a respiratory infection. Indeed, cough may be present in isolation to other symptoms and as the sole manifestation of an episode of asthma.12 Breathlessness may occur as a result of the dynamic lung hyperinflation that accompanies acute asthma episodes and patients may report the sensation of difficulty in “getting air in” their lungs. Exertional symptoms may not be apparent if the patient’s ability to exert themselves is limited by other health conditions such as rheumatologic or cardiac disease and, therefore, asthma may be underdiagnosed in the elderly. No single symptom is specific or more significant for asthma, although wheezing is a useful sign, as nonasthmatics rarely report frequent wheezing. In younger patients, the symptom of chest tightness is helpful, since it occurs more often in association with asthma than with other pulmonary or cardiac disorders. The pattern of symptom occurrence, the precipitating or aggravating factors, and the profile of a typical exacerbation are important elements in the clinical evaluation.
In patients with poorly controlled asthma, symptoms may temporally evolve slowly over days or weeks, or present abruptly. The severity and frequency with which symptoms occur varies greatly within the asthmatic population. The recurrent paroxysmal nature of symptom presentation is characteristic of asthma and symptoms improve, sometimes rather spontaneously, although usually with treatment. Nocturnal episodes are common in adult asthmatics and typically patients awake in the early hours of the morning with symptoms. Distinguishing whether nocturnal symptoms are due to asthma, angina, or gastroesophageal reflux may be difficult, but early-morning asthma symptoms are usually relieved with administration of inhaled bronchodilators, in contrast to cardiovascular symptoms which occur at any time during the night and, gastroesophageal reflux which tends to usually cause symptoms soon after the patient reclines at night.
Chest symptoms that vary by season and are accompanied by symptoms of irritation of other mucus membranes, such as conjunctivitis and rhinitis, are typical of allergic asthma. Triggers such as indoor allergens of house dust mite, cockroach, and animal dander proteins are more likely to result in perennial symptoms, whereas pollens and some mold spores are likely to provoke seasonal symptoms. The presence of rhinosinusitis, nasal polyps, conjunctivitis, or eczema, coupled with a family history of asthma or atopy, may further support the diagnosis of asthma. Symptoms after heavy exertion, especially in the cold air, are highly suggestive of exercise-induced asthma and typically, patients experience symptoms at the end of exercise, rather than during its performance. Excessive coughing after exercise in the absence of wheeze may also be a sign of asthma. Premenopausal women with asthma may experience a deterioration of asthma control perimenstrually.13 The medical history should elicit risk factors for asthma (Table 46-1), and special consideration should address symptoms induced by aspirin or those associated with the patient’s occupation.
 ASTHMA AND ASPIRIN SENSITIVITY
ASTHMA AND ASPIRIN SENSITIVITY
The association of asthma and sensitivity to aspirin or other NSAIDs is well established.14 Aspirin-sensitive asthma affects approximately 5% of all asthmatics, although it is more common in patients with severe asthma (~20%) and in those frequently hospitalized for their asthma. This subtype of asthma is usually characterized by a tetrad of asthma, nasal polyps, chronic hypertrophic eosinophilic sinusitis, and aspirin intolerance. Classically, perennial rhinitis is the first symptom in this syndrome, preceding the development of aspirin sensitivity, and then followed much later by nasal polyps that are usually bilateral and originate from the turbinates and the paranasal sinuses. Even in small doses, aspirin typically causes wheezing, facial flushing, rhinorrhea, and conjunctival irritation. Although aspirin-induced asthmatic episodes often resemble allergic reactions, there is no evidence that immunolglobulin (Ig)-E–related mechanisms are at work. Aspirin-induced asthma is due to blockade of cyclooxygenase 1 by nonsteroidal anti-inflammatory drugs and has been associated with enhanced leukotriene production and mast cell activation, but the cellular pathways responsible for these events remain unclear. The diagnosis of aspirin sensitivity is made on the basis of the clinical history and can be confirmed by a provocative aspirin challenge, although this test carries a potential health risk of anaphylaxis for the patient.
Aspirin-sensitive asthma usually responds to standard therapy with inhaled corticosteroids (ICSs), although the condition is associated with severe asthma, who are a group of patients often refractory to treatment with inhaled and oral CS. Potentially, antileukotriene therapy should be efficacious in these patients, but have been found to be no more effective compared to their use in patients with allergic asthma. Aspirin desensitization may sometimes be needed, and should only be performed in specialized centers. In all asthmatic patients with aspirin sensitivity, the nonselective cyclooxygenase (COX) inhibitors should be avoided, but when an anti-inflammatory analgesic is needed, the selective COX-2 inhibitors are usually safe to use.
Occupational Asthma
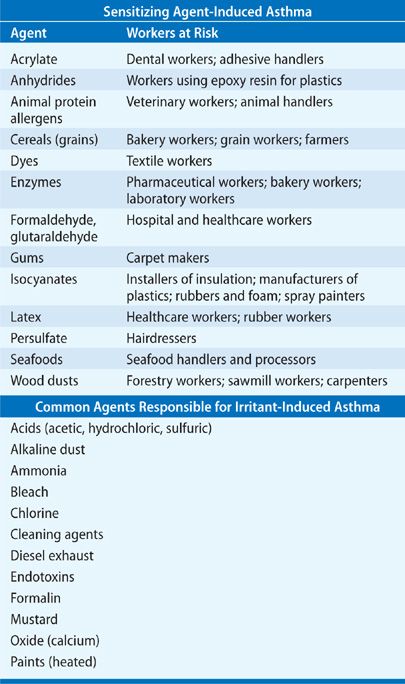
Occupational asthma is asthma arising de novo that is initiated as a consequence of exposure to a specific etiologic agent in people without prior asthma. In contrast, work-exacerbated asthma is defined as the worsening of asthma, that is already pre-existing or concurrent, triggered by nonspecific irritants in the workplace.15 Occupational asthma may be classified into (i) that caused by a sensitizing agent in the workplace (sensitizer-induced asthma) where the specific sensitizing agent causes asthma through an identified underlying immunologic mechanism and (ii) asthma caused by exposure to irritant compounds (irritant-induced asthma) where the exposure agent is not considered to be sensitizing.16 Table 46-2 highlights the causes of both sensitizer-induced occupational asthma and the common agents responsible for irritant-induced occupational asthma. The diagnosis of occupational asthma is based on a demonstrable link between asthma symptoms and workplace exposure, showing work-related variability in measurements of lung function made serially.16 Classically, a typical history of asthma-like symptoms during the working week and improvement over the weekend or on vacation are elicited and symptoms may occur either during exposure to the etiologic substance, or they may be delayed until the evening or night after the work day. Early detection and avoidance of occupational asthma is important where, if the patient is removed from exposure within the first 6 months of symptoms, there is usually complete recovery.
 PHYSICAL EXAMINATION
PHYSICAL EXAMINATION
The most typical physical finding in asthma is wheezing on auscultation, which is usually caused by turbulent airflow through narrowed airways. Wheezing may be heard throughout the chest and is classically polyphonic, present to a greater extent during expiration, although it may also be heard during inspiration. The quality and character of wheezing is not specific to asthma or to the severity of the underlying disease. There may be no abnormal physical findings when asthma is under control yet conversely, in cases of very severe airway obstruction, breath sounds and wheezing may be absent. Examination of the upper respiratory tract may reveal clinical signs of rhinitis, sinusitis, or nasal polyps.
During an acute exacerbation of disease, physical signs of increased ventilation may be observed with the use of accessory muscles of respiration and chest signs of hyperinflation. A sign of severe airway obstruction is pulsus paradoxus, which is the exaggerated decrease in systolic blood pressure during inspiration by >10 mm Hg. As ventilatory effort can be diminished with respiratory muscle fatigue, pulsus paradoxus may be absent, but its absence does not preclude severe airway obstruction. Stridor is a high-pitched inspiratory sound and indicates airflow turbulence in the upper airways. In the acute setting, stridor should prompt a review of causes such as epiglottitis or foreign body, and in chronic presentation conditions such as upper airway tumors, tracheal–bronchial stenosis, vocal cord dysfunction/paralysis, and airway narrowing due to thyroid enlargement should be excluded.
LABORATORY INVESTIGATION
The diagnosis of asthma is usually apparent from the medical history with symptoms of variable and intermittent airway obstruction and objective measurements of lung function and spirometry support the diagnostic process. Similarly, the clinical history provides relevant information regarding the relationship between symptoms and allergen exposure, but skin prick testing and serology may be useful in identifying specific allergic triggers of asthma. Radiologic examination of the thorax, blood tests, and body plethysmography are not routinely indicated, unless there is some uncertainty in the diagnosis, where these tests may be used to exclude other conditions that may mimic asthma or complicate its clinical presentation.
 LUNG FUNCTION TESTS
LUNG FUNCTION TESTS
Peak flow meters are portable devices, readily available for patient use, that measure the peak expiratory flow (PEF). Serial readings of PEF that vary by more than 20% either spontaneously or in response to treatment are supportive of a diagnosis of asthma. Twice-daily PEF measurements, morning and evening, may also demonstrate diurnal variation, which is a typical feature of asthmatic patients.
Spirometry measures the expiratory volume and flow of air using forced maneuvers from full lung inflation, as a function of time. Simple spirometry is important for objectively demonstrating airflow obstruction, confirming the diagnosis of asthma, establishing the severity of the disease, and monitoring the response to therapy. Patients with asthma typically show a reduced forced expiratory flow in 1 second (FEV1), reduced PEF, preserved forced vital capacity (FVC), and an FEV1/FVC ratio of 0.7 or greater, but with worsening disease, FEV1 less than 60% predicted the FEV1/FVC ratio is more usually <0.7.17 Home PEF monitoring may be of diagnostic use, confirming the diurnal variations in airflow obstruction, especially in patients who demonstrate normal spirometry during clinic visits. Spirometry also allows the assessment of the flow–volume loop, which shows a reduced maximum expiratory flow.
Bronchodilator reversibility is a measure of the magnitude of airway smooth muscle relaxation. A postbronchodilator increase in FEV1 of >12% and 200 mL is often considered evidence of reversible airway obstruction, where measures are taken 15 minutes after an inhaled short-acting β2-agonist (SABA). However, this level of increase is arbitrary and lacks sensitivity or specificity for detecting asthma. In addition, bronchodilator reversibility is diminished in well-controlled asthmatic patients, so it is not a good measure of asthma severity or response to therapy. In some patients, bronchodilator reversibility may be demonstrated by a 2- to 4-week trial of oral corticosteroids (prednisone or prednisolone 30–40 mg daily). Bronchodilator reversibility may also occur in patients with chronic obstructive pulmonary disease (COPD), and although asthma and COPD are distinct diseases, an “overlap syndrome” is described between the two conditions.18–20
 BODY PLETHYSMOGRAPHY
BODY PLETHYSMOGRAPHY
Whole-body plethysmography is rarely required to establish a diagnosis of asthma in family practice, but may help in patients where there is diagnostic uncertainty. In stable asthma, measurement of the lung volumes may reveal an increase in residual volume, which reflects airway closure at a lung volume that is higher than normal. Air trapping is typically seen in patients with severe asthma. Airway resistance is characteristically increased and, during acute episodes of disease exacerbation, functional residual capacity and total lung capacity may also be observed to be increased. Measurement of the diffusing capacity of the lung (DLCO) may also differentiate patients with COPD from those with asthma. In stable asthma, DLCO is usually normal, but there may be a small increase in some patients. In contrast, patients with COPD typically have a reduced DLCO, which reflects alveolar septal destruction and loss of pulmonary capillary volume—characteristic features of emphysematous patients.
 BRONCHIAL CHALLENGE TESTING
BRONCHIAL CHALLENGE TESTING
Assessing bronchial hyperresponsiveness (BHR) is a sensitive tool that, although not routinely undertaken in clinical practice, may be helpful in diagnosing asthma, particularly when there is diagnostic uncertainty in the context of normal pulmonary function tests and unexplained chest symptoms (see Chapter 33).21 Bronchial challenge tests assess the abnormally increased airway hyperresponsiveness observed in patients with asthma, by detecting the exaggerated response to inhaled bronchoprovocative agents. The provocation agents can be classified into two categories: direct and indirect. Direct stimuli such as histamine and methacholine, which are normally used in the clinic, act on airway smooth muscle receptors, whereas indirect stimuli act through intermediate pathways that include the release of mast cell mediators, and/or through local and central neurologic reflexes. Indirect stimuli include adenosine monophosphate (AMP), mannitol, exercise, hypertonic saline, and isocapnic hyperventilation.
Increased BHR is typically defined as the inhaled concentration of the bronchoprovocative agent that reduces FEV1 by 20% (PC20). This criterion for the test has maximal sensitivity but not maximal specificity and thus, when a diagnostic PC20 threshold of ≤8 mg/mL is used, pharmacologic challenges are sensitive tests with a high negative predictive value, that is, a PC20 >8 mg/mL excludes a diagnosis of asthma with a high degree of accuracy. Similarly, a positive result, although consistent with is not diagnostic for asthma. False-negative results can be obtained in patients who experience only intermittent symptoms and are tested when they are asymptomatic. The prevalence of abnormal responsiveness in nonatopic, nonasthmatic subjects who have no history of prior respiratory problems ranges between 5% and 10%. Knowledge of family history, personal atopy, and comorbidities clearly improves the prediction that abnormal airway responsiveness predisposes to the subsequent development of asthma.22
Technical factors related to the test procedure must be strictly controlled and follow standard operating procedures that include: the aerosol generation, the method of inhalation (intermittent versus continuous), and the measurement and calculation of the response. Medications such as β2-agonists, theophylline, long-acting muscarinic antagonists, and CSs may influence the test and decrease airway responsiveness. Measuring BHR may have additional utility in the management of asthma. Patients whose disease is considered to be clinically controlled, may still have BHR and underlying airway inflammation and studies have shown that using AHR to guide treatment with ICSs, leads to an additional improvement in symptoms, lung function, and airway biopsy findings, compared with conventional assessment.23
Exercise testing of patients using cycle, treadmill, or free running challenges is occasionally undertaken to show postexercise bronchoconstriction if there is a suggestive history of exercise-induced asthma.24 In professional athletes, asthma may be both under- or overdiagnosed and objective confirmation by appropriate lung function testing with bronchodilator or exercise challenge is often needed. Allergen challenge is rarely utilized in the routine management of patients with asthma and should only be undertaken by a specialist center if a specific causative or occupational agent is to be identified, such as aspirin.
 BLOOD TESTS
BLOOD TESTS
Blood tests are usually not helpful in establishing the diagnosis of asthma. The eosinophil count in the peripheral blood film may be raised in atopic conditions and eosinophilia may support a diagnosis of asthma; however, a normal level does not rule out atopy or exclude asthma. In patients receiving CSs, eosinophilic counts may be normal or low. Because of their poor sensitivity and specificity, blood eosinophil counts are not recommended in the routine monitoring of asthma severity or as a barometer of airway inflammation. Markedly high levels may be present in disorders such as tropical parasitic eosinophilia, allergic bronchopulmonary aspergillosis (ABPA), Churg–Strauss syndrome, and Loeffler’s syndrome as discussed elsewhere in this volume. In these hypereosinophilic conditions, clinical suspicion may warrant additional blood tests directed to ruling out vasculitis or ABPA, which are uncommon causes of asthma symptoms.
Total serum immunoglobulin E (IgE) may be measured in patients. Epidemiologic studies demonstrate an association between asthma and total serum IgE levels, standardized for sex and age. There is also a relationship between total serum IgE and asthma in patients with negative skin tests. Importantly, total IgE levels are used to calculate the dose of the anti-IgE antibody therapy, omalizumab, when it is used for asthma treatment as discussed below in Anti-IgE Monoclonal Antibodies. Blood tests of specific IgE to inhaled allergens, radioallergosorbent testing (RAST), and immunoCAP may help identify or confirm allergy to specific allergens, such as house dust mite, cockroach, Aspergillus species, pollens, or animal dander.
In acute exacerbations of disease, arterial blood gases may reveal hypoxemia and the arterial PaCO2 may be reduced due to hyperventilation. With a severe exacerbation, the arterial PaCO2 may rise due to respiratory muscle fatigue and an inability to maintain the required alveolar ventilation.
 SKIN TESTS
SKIN TESTS
If the clinical history suggests specific aeroallergens are important triggers or when asthma symptoms in a patient are accompanied by other symptoms typical of allergic disease, such as conjunctivitis or rhinitis, skin prick tests may be helpful to determine whether the patient is allergic, and to investigate the role of specific allergens as a cause of asthma. Sensitivity to a particular allergen such as house dust mite, cockroach, Aspergillus species or animal dander can be verified by skin tests or in vitro serum antibody studies (see above). Antihistamines and antidepressants should be avoided when undertaking testing as these drugs can interfere with the response. Positive responses on skin prick testing may help encourage patients to undertake allergen avoidance measures or, in selected cases, may help develop immunotherapy regimens.
 CHEST IMAGING
CHEST IMAGING
Chest radiography is usually unremarkable and normal in patients with mild-to-moderate asthma; however, in more severe disease, nonspecific findings such as hyperinflation, prominent hilar vessels, and bronchial wall thickening may be seen. In patients with an exacerbation of their symptoms, chest radiography may be useful to exclude a pneumothorax. Consolidation shadowing in the lung usually indicates pneumonia or eosinophilic infiltrates in patients with ABPA. High-resolution computed tomography (HRCT) of the chest may identify atelectasis, bronchial wall thickening, or areas of bronchiectasis in patients with severe asthma, but these changes are not diagnostic of asthma. Emphysema is absent. Multidetector computed tomography (MDCT) undertaken in inspiration and expiration provides additional information concerning the tracheobronchial tree during the entire respiratory cycle.
 EXHALED NITRIC OXIDE
EXHALED NITRIC OXIDE
The measurement of fractional nitric oxide gas in the exhaled breath (FeNO) of patients is being utilized as a noninvasive test to assess intrapulmonary eosinophilic inflammation.25 Portable, compact hand-held devices allow FeNO measurements to be undertaken at the bedside and in family practice. Typically, asthmatic patients have elevated FeNO levels compared with healthy subjects, which correlate with the amount of eosinophils in sputum. ICSs and oral leukotriene receptor antagonists have been shown to decrease FeNO levels. These observations suggest a possible role for FeNO as an index of asthma disease severity, as a test of treatment efficacy and, in the assessment of patient adherence with asthma therapy. Measurements of FeNO have also been used successfully to titrate inhaled steroids without any loss of asthma control; thus, FeNO may be used as a tool in conjunction with other clinical measures to optimize asthma management as recommended by guidelines, that is, achieving disease control using the lowest doses of medications possible. In the research environment, FeNO can be partitioned into that arising from the central bronchial/conducting airways, or to that generated in peripheral alveolar regions, allowing an assessment of the site of intrapulmonary inflammation.26,27 Patients with severe refractory asthma have shown greater alveolar NO concentrations compared to those with mild asthma.
 SPUTUM EXAMINATION
SPUTUM EXAMINATION
The sputum differential count may be helpful. Induced sputum eosinophil counts have been used as an endpoint in clinical trials of therapeutic agents targeted at patients with eosinophilic lung diseases like asthma.28 Research studies have shown sputum eosinophilia predicts clinical outcomes, particularly asthma exacerbations, when CSs are withdrawn. Induced sputum eosinophil counts have also been shown to guide anti-inflammatory treatment in patients with asthma in a management strategy that minimizes eosinophilic inflammation.23 However, induced sputum remains a research tool as it is rather an unpleasant procedure for the patient and further studies are needed before measurement of sputum eosinophils can be widely used as a biomarker to monitor patients in clinical practice.
DIFFERENTIAL DIAGNOSIS
There are a number of conditions to consider in the differential diagnosis of asthma and these are listed in Table 46-3. Usually, it is not difficult to differentiate asthma from other conditions causing wheeze and dyspnea. The degree of diagnostic accuracy is probably dependent on the age of the patient, where the diagnosis in young adults is usually not difficult since there are few other conditions that mimic asthma or confound its clinical presentation. With increasing age, cardiovascular disease and other forms of chronic lung disease are more common, and the differential diagnosis of episodic chest symptoms is broader.
Patients with upper airway obstruction can mimic severe asthma, and typically these patients present with localized wheeze and stridor of the large airways. Assessing the flow–volume loop in such patients will reveal a reduction in inspiratory flow as well as expiratory flow, and bronchoscopy can demonstrate the site of narrowing in the upper airways. Vocal cord dysfunction can be assessed using nasoendoscopy, which allows the observation of abnormalities in the movement of the vocal cords, and is most helpful when adduction of the cords is detected in the presence of the patient’s symptoms.29 Persistent wheezing auscultated in a localized area of the chest wall may indicate endobronchial obstruction due to lung cancer or a foreign body. Eosinophilic pneumonias and systemic vasculitis, including the Churg–Strauss syndrome and polyarteritis nodosa may be associated with wheezing and their systemic clinical manifestations may help in their identification.
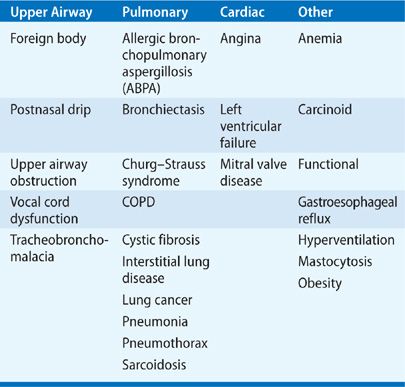
COPD is usually easy to differentiate from asthma. The symptoms in patients with COPD are more persistent, show less variability, are progressive, and usually exhibit minimal reversibility to bronchodilator agents. The literature highlights an “overlap syndrome,” where COPD patients have features of asthma with increased sputum eosinophils and a response to oral corticosteroids; these patients probably have both diseases concomitantly.20 Important cardiologic causes to consider include left ventricular failure, where usually bibasal lung crackles are present in contrast to the scattered polyphonic wheeze in asthma. Anemia should always be thought of as a cause of dyspnea, especially in elderly patients. The symptoms of gastroesophageal reflux disease (GERD) may be mistaken for those of asthma; however, it is important to recognize that GERD is common in patients with asthma and has been identified as a potential trigger for asthma symptoms.30
TREATMENT OF ASTHMA
Treating asthmatic patients is generally straightforward; with effective and safe drugs, most asthmatics are now managed by family doctors. The successful management of asthma requires an appreciation of the heterogeneity of the disease with respect to etiology, clinical presentation, severity, natural history, and response to therapy. It is unlikely that a single management approach will work for all patients and hence, treatment should be tailored to the individual patient. It will also be recognized that symptom severity in patients varies over time with periods of remission that are interspersed with acute exacerbations, and thus the patient should be monitored regularly and treatment should be modified on an ongoing basis to meet the patient’s current needs. There are several aims in the management of patients with asthma (Table 46-4) and although prominence has been placed on drug therapy, there are important patient-orientated approaches that focus on correct inhaler usage, emphasize self-management action plans, and address environmental control.



