Assessing and Improving the Quality of Care in Cardiovascular Medicine: Introduction
Quality is never an accident; it is always the result of high intention, sincere effort, intelligent direction and skillful execution; it represents the wise choice of many alternatives.
—Willa A. Foster
In the United States, health care expenditures account for nearly 16% of the gross domestic product and are continuing to rise.1 Although many technologic and therapeutic advances are worthy of praise, the quality of care delivered is nowhere near the level expected or desired from our investment.2-4 Cardiovascular medicine, in particular, has benefitted enormously from the investment in scientific discovery and clinical research5; yet the timely, systematic translation of new knowledge into clinical practice remains a challenge. To advance evidence-based practice and to reduce the variability in the quality of cardiovascular care, three key tools—clinical guidelines, performance measures, and appropriate use criteria—have been developed and promoted for quality assessment and improvement. This chapter provides an overview of these tools in the context of accepted frameworks to maximize quality and safety, after briefly reviewing the need for continual assessment and improvement of the quality of health care in the United States.
Cardiovascular Care in the United States
The United States invests an estimated $2.4 trillion dollars in health care, with a projected annual growth rate of 6.2% over the next 10 years.1 For a country that spends more money than any other nation in the world,6 however, the United States ranks poorly on most standardized health indices7 and quality metrics.4,8 In the United States, cardiovascular disease (CVD) remains the leading cause of death and disability, with an estimated annual total cost of $475.3 billion.9 Such a magnitude of disease burden necessitates and demands careful scrutiny of the quality of care being delivered.
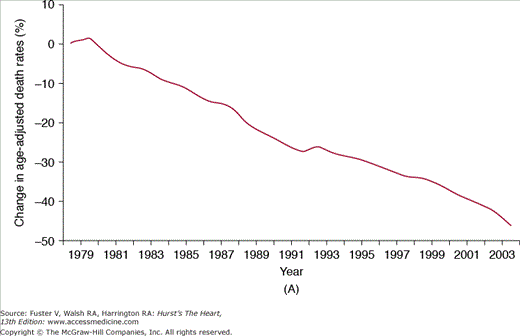
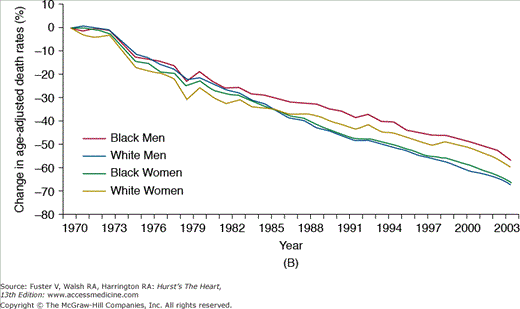
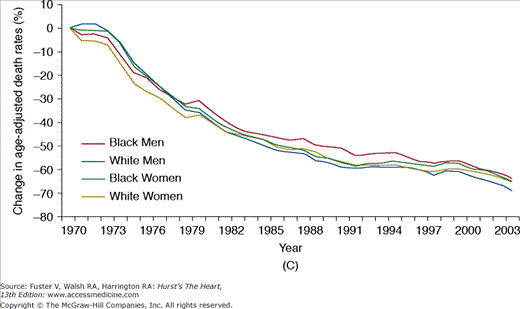
Cardiovascular medicine in the United States has benefitted enormously from the investment in scientific discovery and clinical research, leading to many advances in the knowledge and treatment of the disease process. Importantly, the last 3 decades have witnessed substantial improvements in age-adjusted mortality rates due to CVD, coronary artery disease (CAD), and stroke (Fig. 3–1),5 a likely reflection of the successful adoption of primary and secondary prevention, coupled with improved treatments for acute cardiac conditions. Data from large population studies support these findings with marked reductions observed in the age- and race-adjusted rates of sudden death due to CAD,10 as well as in the incidence, case fatality rates, and mortality associated with CVD between 1971-1982 and 1982-1992.11a-11c
FIGURE 3–1
Percent decline in age-adjusted mortality rates for (A) cardiovascular disease (reference year: 1979), (B) coronary artery disease (reference year: 1970), and (C) stroke (reference year: 1970): United States. Death rates are age-adjusted to the 2000 US census population. Reproduced with permission from the National Institutes of Health: National Heart, Lung, and Blood Institute.5
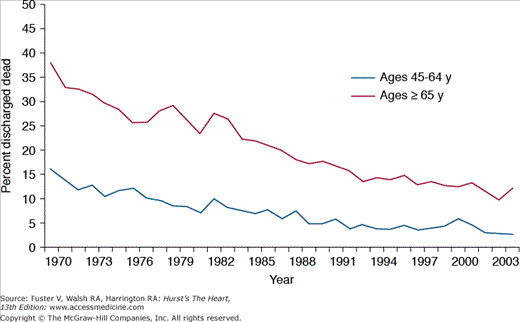
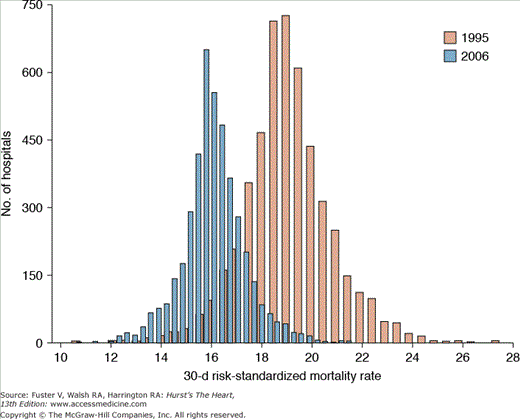
Beyond merely developing new diagnostic and therapeutic modalities, cardiovascular professionals have also led highly visible local and national initiatives to promote the use of evidence-based medicine in clinical practice.12-16 Acute myocardial infarction (AMI), in particular, has been at the forefront of quality initiatives, with multiple studies examining trends in outcomes and patterns of care over time.13,17,18 Temporal trends in AMI outcomes reflect significant reductions in the number of adult hospitalizations for heart attacks,19 associated case fatality rates (Fig. 3–2),5 and overall mortality.20 To explore potential reasons for these trends and to develop insights into actionable opportunities to improve outcomes, several studies have evaluated the use of evidence-based processes of care in AMI. One of the first insights came from the Cooperative Cardiovascular Pilot Project, a four-state study of approximately 17,000 AMI patients.21 This report documented significant underuse of potentially life-saving therapies in the setting of a myocardial infarction (MI) and highlighted an important opportunity to improve the application of evidenced-based care in routine clinical practice. Since then, AMI care has dramatically improved with enhanced use of guideline-based therapies17 and marked reductions in risk-standardized hospital mortality rates22 and interhospital variations.22,23 The most recent analyses of outcomes in the Medicare population identified two important points surrounding the improvements of care between 1995 and 2006. First, there has been a clear reduction in the mean 30-day, risk-standardized mortality rates after AMI from 18.8% to 15.8%, an absolute reduction of 3% (Fig. 3–3).22 This means that, on an average, one more patient of every 33 admitted in 2006, as compared with 1995, are now alive 30 days after their event. Second, there has been a notable narrowing of the distribution of mortality across hospitals, meaning that more homogenous care and outcomes are being achieved throughout the country.23 These developments are critical and underscore the systematic efforts of the profession in achieving meaningful improvements in outcomes throughout the United States. However, it must be noted that despite these successes, multiple studies have elaborately documented marked inconsistencies in cardiovascular care across geographic regions3,23-26 and in patient risk status.27-32 Moreover, other highly prevalent conditions, such as heart failure, have lagged behind in meeting quality standards observed in AMI care.23,25,33 Collectively, these variations, some examples of which are reviewed in the subsequent section, underscore the Institute of Medicine’s (IOM’s) thesis that “there is not a gap between what the health care is now and what could be, but rather a chasm.”34
FIGURE 3–2
Temporal trends in hospital case fatality rates for acute myocardial infarction, ages 45-64 and 65 and over, United States, 1970-2004. Death rates are age-adjusted to the 2000 US census population. Reproduced with permission from the National Institutes of Health: National Heart, Lung, and Blood Institute.5
FIGURE 3–3
Change in acute myocardial infarction 30-day all-cause risk-standardized mortality from 1995 to 2006. Used with permission from Krumholz et al.22 Copyright © 2009 American Medical Association. All rights reserved.
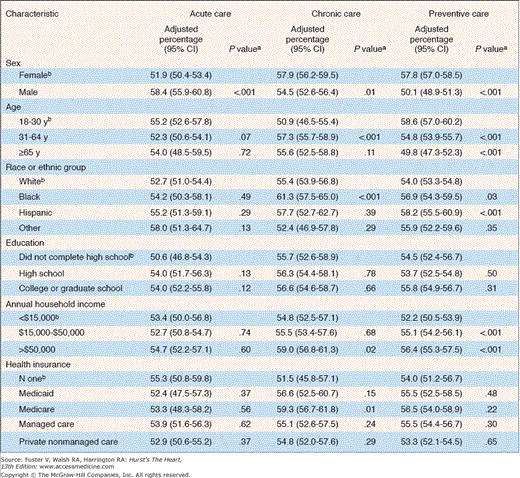
A critical goal of efforts to disseminate high-quality care is to ensure rational and efficient use of effective treatment to those who derive the most benefit.34 Yet, surveys evaluating processes of care have shown that, on an average, only one in two US adults gets recommended care when receiving health care services.3,31 Adherence to recommended processes of care, identified using 439 quality indicators for 30 acute and chronic conditions, suggest marked variations in the use of evidence-based treatments as a function of sex, age, race, education, income, and insurance status (Fig. 3–4).3 These differences in the processes of care run counter to established ethical principles of equity and underscore the need for tools to systematically elevate the quality of care for all.
FIGURE 3–4
Adjusted percentage of recommended care received by participants, according to characteristic and type of care. The percentages have been adjusted for all variables listed in the table in addition to self-reported health status, number of visits to a health care provider, number of hospitalizations, number of acute and chronic conditions during the study period, and metropolitan statistical area. CI, confidence interval. a P values were calculated after sampling and nonresponse weights had been applied. bThis group of participants was used as the comparison group in the multivariate analysis and the reference category for the calculation of P values. Data from Asch et al.31 Copyright © 2006 Massachusetts Medical Society. All rights reserved.
Beyond concerns about overall disparities in care, there is emerging evidence that among the patients eligible for treatments, those with the least potential to benefit are preferentially treated whereas those with the most to gain are systematically undertreated. Referred to as the risk-treatment paradox, numerous investigators have shown that high-risk patients—who would be expected to benefit more than lower risk patients—are preferentially treated less aggressively, whereas lower risk patients are treated more aggressively.27-30 For example, patients with non–ST-segment elevation acute coronary syndrome identified as being at high risk for in-hospital mortality were less likely to be treated with guideline-recommended therapies than patients classified as low or medium risk.32 The use of angiography and angioplasty in AMI survivors was inversely correlated with most clinical factors that predicted high mortality risk.27 Furthermore, as demonstrated by Pilote and colleagues,28 younger age and the availability of an on-site angioplasty facility emerged as stronger determinants for angiography use than guideline-recommended indications of recurrent ischemia or cardiogenic shock. Underscoring this illogical use of angiography and revascularization, data from registries such as the Myocardial Infarction Triage and Intervention Project35 and the Cooperative Cardiovascular Project36 showed no survival or economic benefit for AMI patients admitted to hospitals with, versus without, on-site cardiac catheterization facilities.
Further emphasizing the need to monitor the quality of care has been the observation of marked variations in the processes of care by geographic region. Pioneering work from the Dartmouth Atlas series, a comprehensive evaluation of health care services provided to Medicare beneficiaries, has documented broad variation in the use of both diagnostic and treatment modalities in CVD as a function of the site of care.37 In the first Dartmouth Atlas of Cardiovascular Health Care, Wennberg and colleagues found wide variations in the use of echocardiograms, ranging from 56 per 1000 beneficiaries in Portland, Oregon to 339 per 1000 beneficiaries in Miami. Considerable differences were also observed in the use of angioplasty, with rates ranging from <3 to >20 per 1000 patients, after adjustment for age, sex, and race. Ko and colleagues29 further extended this work in a distinct cohort of Medicare enrollees to show that the use of invasive therapy after AMI was independent of the patient’s baseline risk status or the appropriateness of the procedure.
These patterns of unexplained variation extend into other processes of care and range from acute events, such as resuscitating patients experiencing a cardiac arrest, to increasingly prevalent chronic conditions, such as heart failure. For example, Chan and colleagues,26 using data from 200 hospitals in the National Registry of Cardiopulmonary Resuscitation (NRCPR), have shown that the adjusted rates of delayed defibrillation, defined as >2 minutes from the recognition of cardiac arrest, ranged from 2.4% to 50.9% across participating hospitals. These variations were associated with marked differences in survival to hospital discharge.
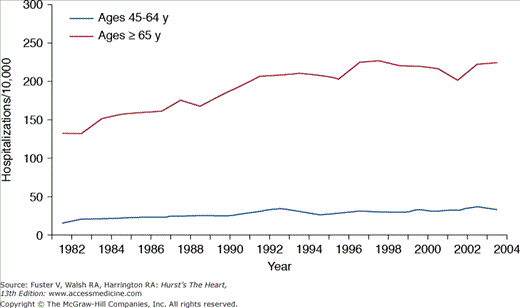
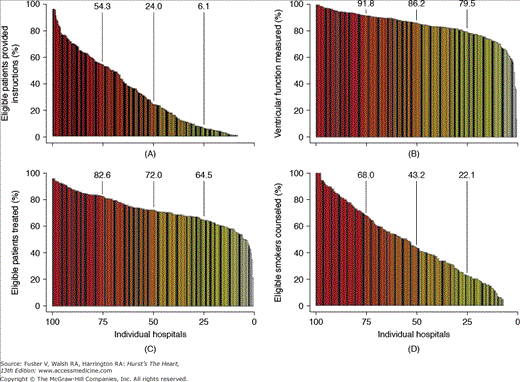
Heart failure, an increasingly prevalent condition in the United States, imposes a substantial clinical and economic burden on our health care system.9,38 In the last 2 decades, hospitalization rates for heart failure have increased dramatically in individuals aged 45 to 64 years and those aged ≥65 years (Fig. 3–5)9 but with no comparable improvements in readmission or all-cause mortality rates.33,39 National registries have also documented significant, persistent interhospital variability in the care provided for patients with heart failure (Fig. 3–6).23,25,40
FIGURE 3–5
Temporal trends in hospitalization rates for heart failure, ages 45-64 and 65 and over. Death rates are age-adjusted to the 2000 US census population. Reproduced with permission from the National Institutes of Health: National Heart, Lung, and Blood Institute.5
FIGURE 3–6
Frequency distribution of conformity rates by hospital for the Joint Commission core performance measures (A) HF-1 (discharge instructions or guidance), (B) HF-2 (left ventricular function documentation obtained or scheduled), (C) HF-3 (angiotensin-converting enzyme inhibitor prescribed at discharge for left ventricular systolic dysfunction), and (D) HF-4 (smoking cessation counseling, if indicated). Each bar represents an individual hospital; vertical lines above bars represent the 25th, 50th, and 75th percentiles. Reproduced with permission from Fonarow et al.40 Copyright © 2005 American Medical Association. All rights reserved.
Thus, repeated documentation of widespread over-, under-, or even misuse of health care resources, coupled with an absence of data supporting favorable differences in outcomes, challenge the current practice of health care in the United States and mandate efforts to improve the quality of care. Such variations in care, based on sociodemographic factors, clinical risk, and geographic regions, highlight the breadth and depth of the problem and mandate improved approaches to systematically and equitably apply care. As such, these gaps are the rallying cries for the standardized monitoring of the quality of care with the goal of ensuring appropriate, evidence-based, patient-centered care.
Frameworks for Assessing the Quality of Care
Lohr and Schroeder41 broadly define quality of care as “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge.” The US Agency for Healthcare Research and Quality42 has proposed a similar definition: “Quality health care means doing the right thing at the right time in the right way for the right person and having the best results possible.” Although the concept of quality health care is intuitive and relatively straightforward to understand, to actually measure, monitor, and improve quality necessitates the use of a clear conceptual framework that encompasses important relevant aspects of health care.
Conceptualizing quality is critically dependent on the perspective of the observer. This, in turn, influences the approach to quality assessment (QA), namely, determining who is being assessed, what processes are being monitored, and what expected outcomes are being evaluated. Health care is a complex multidimensional phenomenon with a myriad of factors influencing both the delivery and quality of care provided. Multiple organizations have undertaken initiatives to quantify delivery and quality of care and, in so doing, have pursued different goals, perspectives, and tools for assessing quality. The following sections highlight the two most common approaches for performing QA/quality improvement (QI) initiatives.
One of the earliest approaches to conceptualizing the components of QA was proposed by Donabedian.43 This framework considers quality as being comprised of three main domains: structure, process, and outcome. Structure refers to the attributes of settings where care is delivered and includes aspects that exist independently of the patient. Examples of structural attributes include provider training and experience, availability of specialized treatments, nurse-to-patient ratios, and treatment and discharge plans. Process refers to whether or not good medical practices are followed and incorporates concepts such as the medications given and timing of their administration, the use of diagnostic and therapeutic procedures, and patient counseling. Outcome refers to tangible measures that capture the consequences of care and range from manifestations of disease progression to patient-centered outcomes of health status and treatment satisfaction. As noted by Donabedian, these three components of quality are interdependent and built on a framework that primarily focuses on the delivery of care that aids in linking delivery to outcomes.
The current driving force and roadmap for QI initiatives in American health care is the IOM’s landmark report, “Crossing the Quality Chasm: A New Health System for the 21st Century.”34 In this report, the IOM recognized substantial deficiencies in the current status of American medicine, while concurrently recognizing that individual practitioners were deeply committed to providing high-quality care. They thus focused on the need for a major overhaul of the health care system with a view to placing the patient at the center of QI initiatives. The IOM recognized the following principal thematic dimensions needed to guide QI in health care:
- Safety—avoiding injuries to patients from the care that is intended to help them.
- Effectiveness—providing services based on scientific knowledge to those who could benefit, while refraining from providing services to those not likely to benefit.
- Patient-centeredness—providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.
- Timeliness—reducing waits and sometimes harmful delays in care.
- Efficiency—avoiding waste, including waste of equipment, supplies, ideas, and energy.
- Equity —providing care that does not vary in quality because of personal characteristics such as sex, ethnicity, geographic location, and socioeconomic status.
Both the Donabedian components and the IOM principles are landmark frameworks that have helped steer the health care system in the United States toward betterment of the quality and safety of care. Importantly, although no explicit map for achieving better care was defined, a number of well-developed goals and rules were articulated to serve as guides to reorganizing the health care system. To better understand the use of QA tools, we describe a prototypical framework that combines the IOM principles and the Donabedian components in the context of the delivery of health care (Fig. 3–7). Consider a patient who enters the health care system for management of an AMI. Delivery of care extends from the initiation of prehospital care by emergency medical services to treatment and posthospitalization follow-up. The structural component of QA/QI comprises the systems responsible for the provision of care, the material resources on which those systems depend, and the organizational structures that guide their interactions. Patient-care systems include the prehospital services, the emergency department, inpatient resources, and the outpatient system to which the patients are referred for postdischarge care (eg, availability of cardiac rehabilitation). Material resources refer to the personnel (their number, training, and competence) and equipment available for patient treatment, and organizational systems
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree