Aspiration, Empyema, Lung Abscesses, and Anaerobic Infections
OVERVIEW
Aspiration pneumonia, lung abscess, and necrotic lung are parenchymal lung diseases. Aspiration pneumonia refers to the pulmonary consequences that follow abnormal entry of fluid, particulate substances, or endogenous secretions from the upper airways or gastric contents into the lower airways (see also Chapter 69). To develop aspiration pneumonia, a series of formidable host defense mechanisms that normally protect the lower airways must be overcome, including glottic closure via the cricopharyngeus muscle, the cough reflex, ciliary clearance, and other defense mechanisms. The material aspirated must generate an inflammatory response or cause obstruction. The nature of the pneumonia that develops depends on the inoculum and the host response. Anaerobic bacteria are the most common pathogens in this setting, reflecting both pathogenic potential and importance in the normal flora of the upper airways. Risk factors for aspiration may be transient (anesthesia, intoxication) or persistent (e.g., neuromuscular disorders, achalasia) with the risk for recurrence depending on recognition and resolution of the inciting defect.1,2
Lung abscesses reflect infection with an unusual microbial burden (e.g., acute aspiration), a failure in microbial clearance mechanisms (e.g., bronchial obstruction), or both, leading to necrosis of pulmonary tissue and formation of cavities containing necrotic debris or fluid (Fig. 127-1). The formation of multiple smaller (less than 2 cm) abscesses in pulmonary tissue is occasionally referred to as necrotizing pneumonia or lung gangrene. Both lung abscess and necrotizing pneumonia are manifestations of the same pathologic processes, and the distinction is, therefore, arbitrary.3
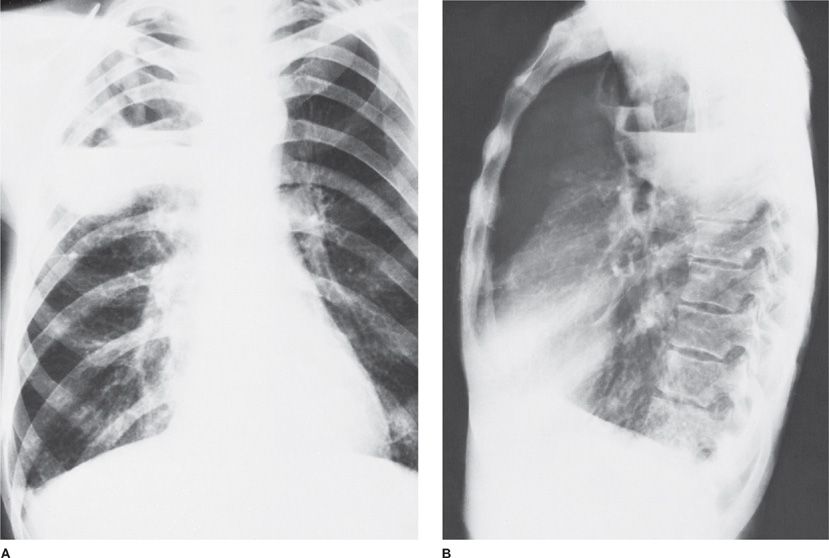
Figure 127-1 A. Anaerobic pneumonia with abscess formation in a 48-year-old alcoholic man. The abscesses are located in the posterior segment of right upper lobe, a dependent segment that is seen best on lateral view (B).
Empyema refers to a purulent collection in any body site but is commonly used to indicate infection of the pleural space.4 Empyema is typically associated with underlying pulmonary parenchymal infection but may also be associated with blood-borne infection, thoracic surgery, trauma, abdominal infection, or neoplasm.3 Failure to recognize and treat either empyema or lung abscess is associated with a poor clinical outcome.5,6 In the preantibiotic era, lung abscess was associated with a mortality approaching 40%.7 However, controversy exists over the best approaches to both processes in terms of antimicrobial selection and physical drainage.5,6
HISTORY
In 1893, Veillon8 published a review of “fetid infections,” first marking the published record of infections due to anaerobic pathogens. However, anaerobes are now largely forgotten potential pathogens in pulmonary infection, including in both community- and healthcare- associated pneumonia.1 The clinical and bacteriologic features of anaerobic infections of the lung have been documented by extensive studies during two periods of investigation.1 The first was at the turn of the century, when anaerobic bacteria were initially reported as important causes of empyema. This early work continued through the late 1920s, when David Smith conducted classic studies on the pathogenesis of lung abscess. At that time, approximately one-third of patients with lung abscess died. Smith noted that the bacteria in the walls of the abscess at autopsy resembled the bacteria found in the gingival crevice, leading him to conclude that aspiration was the major mechanism in pathogenesis. He subsequently supported this hypothesis by inoculating the trachea of experimental animals with gingival crevice material to reproduce the sequence of events of pneumonitis, followed by lung abscess formation in 7 to 10 days.9 Bacteriologic studies of the inoculum showed that four bacterial species were critical, and all were anaerobic bacteria: a fusiform bacterium now recognized as Fusobacterium nucleatum, Prevotella melaninogenica (formerly Bacteroides melaninogenicus), Peptostreptococcus, and an anaerobic spirochete. This study is one of the first demonstrations of bacterial synergy, defined as the demonstration that two or more bacterial species are required to produce a pathologic process that could not be reproduced by any single component of the inoculum.9
In the first two or three decades of the antibiotic era, the role of anaerobic bacteria in this and other pathologic processes was largely ignored. Patients with lung abscesses often had putrid sputum and no identifiable pathogen; these infections were frequently referred to as nonspecific lung abscess. Although the microbial cause was unknown, it was well established that these patients almost invariably responded to penicillin treatment. The role of anaerobes in empyema was also largely ignored. Much of this neglect is ascribed to the paucity of laboratories capable of cultivating oxygen-sensitive bacteria. Studies of anaerobic bacteria were spawned by the ability to culture anaerobes in clinical laboratories with the introduction of anaerobic gas–generating culture jars, the description of the taxonomy of these organisms, and the availability of new antimicrobial agents (clindamycin, metronidazole, cefoxitin) for the therapy. The introduction and widespread use of transtracheal aspiration (TTA) in the late 1960s made it realistic to collect uncontaminated specimens from the lower airways that could be used for anaerobic cultures. TTAs are seldom performed today, so anaerobic bacteria are rarely established as pulmonary pathogens. These organisms are often suspected on the basis of the etiologic route of infection (e.g., oropharyngeal flora) and their importance in patients with aspiration pneumonia, necrotizing pneumonia, lung abscess, and empyema.1
PATHOPHYSIOLOGY OF ASPIRATION, EMPYEMA, AND LUNG ABSCESS
The bacteria implicated in anaerobic lung infections represent the normal flora of the oral cavity—primarily the gingival crevice, where anaerobic bacteria are found in concentrations that approach the geometric limits with which bacteria occupy space: 1012/g.10 Compromised consciousness or dysphagia is the most frequently predisposing risk factor for clinically significant aspiration. Common conditions associated with clinically significant aspiration include alcoholism, general anesthesia, seizure disorder, drug abuse, esophageal lesions, hepatic failure with decreased level of consciousness, and neurologic deficits associated with dysphagia.11
Additional conditions that appear to predispose to anaerobic infections include pulmonary infarction, obstruction due to carcinoma or a foreign body, and bronchiectasis. These conditions are associated with stasis or necrosis of tissue, which presumably accounts for the association with anaerobic infections. The use of acid suppressing medication has also been associated with community-acquired, hospital-acquired, and ventilator-associated pneumonia, presumably due to loss of the gastric acid barrier to bacterial growth.12–14
A somewhat unique feature of anaerobic lung infections is the proclivity for necrosis of tissue, resulting in abscess formation or a bronchopleural fistula associated with empyema.15 Virulence factors of anaerobic bacteria presumed to account for this association include the capsular polysaccharide of anaerobic gram-negative bacilli. The most extensively studied is the polysaccharide of Bacteroides fragilis, but the same observations may apply to P. melaninogenica and other anaerobic gram-negative bacilli.16,17 The capsule of B. fragilis consists of a family of polysaccharides composed of oligosaccharide repeating units with sugars containing positively charged free amino groups and negatively charged carboxyl, phosphate, and amino groups.18–20 These positive and negative charges mediate the capacity to induce abscess formation in experimental animals.21,22 Another virulence factor possessed by most anaerobic bacteria is the production of short-chain fatty acids that inhibit phagocytic killing at low pH levels.23,24 Short-chain volatile fatty acids are metabolic products of anaerobic bacteria that are used to classify these organisms taxonomically, and they appear to be responsible for the putrid odor that is often a characteristic feature of infections by these organisms.25,26
MICROBIOLOGY
Bacteriologic findings of anaerobic lung infections from two large series are summarized in Table 127-1. Most of these infections involve multiple bacterial species, and approximately half of the patients have anaerobic bacteria combined with potentially pathogenic aerobic or facultative anaerobes. Analysis of community-acquired infections involving only anaerobes versus those that are mixtures of aerobic and anaerobic bacteria shows common clinical features with no difference in terms of the frequency of suspected aspiration, indolent presentation, or the frequency of putrid discharge. The implication is that a putrid lung abscess with Escherichia coli in expectorated sputum or anaerobic bacteria plus E. coli in a TTA should usually be considered an anaerobic infection. Caution is advised in applying these conclusions to nosocomial pulmonary infections, since this is a setting in which the aerobic component of the infection is probably more important.
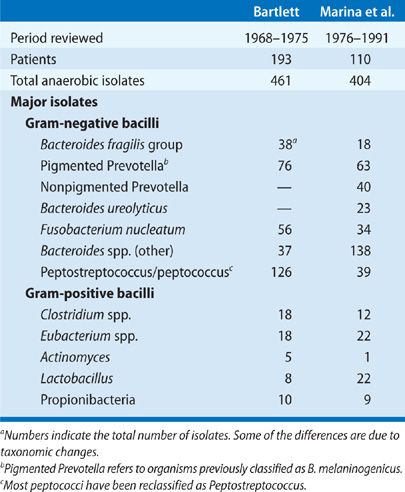
The establishment of anaerobic bacteria in pulmonary infections requires specimens of respiratory secretions that are devoid of contamination from the upper airways. The usual procedures satisfying this criterion are TTA, transthoracic aspiration, open lung biopsy, thoracentesis, and more recently, bronchoscopy with quantitative cultures. In addition, there must be appropriate laboratory expertise for the cultivation of anaerobic bacteria. The incidence of anaerobic lung infections reported in published studies from the antibiotic era that satisfy both requirements is summarized in Table 127-2.
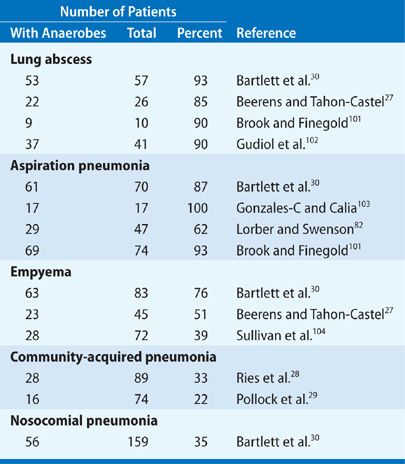
Most published reports deal with the role of anaerobic bacteria in aspiration pneumonia or lung abscess, and these show recovery rates ranging from 62% to 100%. The usual specimens in these studies are TTA and transthoracic aspiration. One of the best studies is by Beerens and Tahon-Castel,27 who used transthoracic needle aspiration to characterize the flora in lung abscesses; this series showed recovery of anaerobic bacteria, usually in pure culture, in 22 (85%) of 26 cases.
The major bacterial isolates in patients with anaerobic lung infections are Peptostreptococcus, F. nucleatum, and P. melaninogenica. Aerobic and microaerophilic streptococci are commonly present and may contribute to the pathogenic events. At least 15% to 25% of anaerobic bacteria responsible for lung infections are resistant to penicillin, generally due to penicillinase production. These susceptibility data are rarely available in individual cases unless specifically requested.
There have been few studies to identify the frequency of anaerobic bacteria in unselected cases of community-acquired pneumonia. One was by Ries et al.,28 who performed TTAs in patients hospitalized with a diagnosis of pneumonia and recovered anaerobic bacteria in 29 (33%) of 89 cases. A more recent study by Pollock et al.,29 using fiberoptic bronchoscopy with a protected catheter and quantitative cultures, showed recovery of anaerobes in 16 (22%) of 74 patients. These two reports suggest that anaerobic bacteria are relatively common pathogens among patients with community-acquired pneumonia and presumably account for a substantial proportion of cases that are now considered enigmatic. In nosocomial pneumonia, a study by Bartlett et al.30 utilized TTA in 159 consecutive patients and showed anaerobes in 56 (35%). Nevertheless, most of these patients also showed the concurrent presence of aerobic gram-negative bacilli or Staphylococcus aureus, and their clinical course was determined largely by the aerobic pathogens.
CLINICAL FEATURES
Nearly all patients with anaerobic lung infections have the usual constitutional findings for patients with infection (Table 127-3). A review of 193 bacteriologically confirmed cases showed that the mean peak temperature for hospitalized patients was 39.1°C and all but five patients were febrile. The average peripheral leukocyte count was 15,000/mL3. Patients who presented with the suppurative complications had a longer duration of symptoms before presentation; this was commonly associated with other evidence of chronic disease, including weight loss and anemia. Another common feature of patients with suppurative complications was putrid sputum or empyema fluid, which was noted in 40% to 60%. It should be emphasized that the putrid discharge in these cases is considered diagnostic of anaerobic infection, since aerobic bacteria are not capable of producing this characteristic odor either in vitro or in vivo. Thus, anaerobic bacteria may cause a diverse range of pulmonary infections, which may be acute, subacute, or chronic. The anaerobic etiology is rarely established or even suspected in patients with acute pneumonitis. This compares with aspiration pneumonia, where anaerobes are presumed pathogens in most community-acquired infections and may contribute to many nosocomial cases. By contrast, anaerobic bacteria are readily recognized as probable pathogens in patients who have the late suppurative complications, such as lung abscess or empyema.
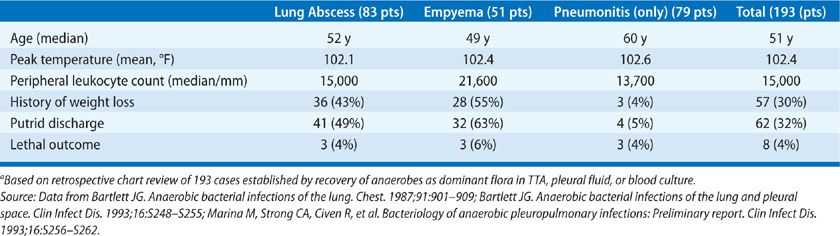
One review of 46 patients with anaerobic bacterial pneumonitis showed clinical features that were similar to those of pneumococcal pneumonia.31 The diagnosis was established by TTA, and the results in this group were compared with those in a second group of patients in whom TTAs yielded Streptococcus pneumoniae. The two groups were similar in terms of age, changes on the chest radiograph, peak temperature, and peripheral leukocyte count. Significant differences in the group with anaerobic infections were the lack of rigors, a somewhat longer duration of symptoms before presentation, and a more frequent association with predisposing conditions for aspiration.31 An important point to emphasize is that patients seen in this early stage of infection rarely have the features that are commonly associated with anaerobic lung infections, such as putrid sputum, tissue necrosis with abscess formation, or a chronic course. These infections presumably account for some and possibly many of the cases of community-acquired pneumonia in which no etiologic diagnosis is established despite extensive study; such cases account for 40% to 50% of cases in most series. Features of anaerobic infections that are nearly unique are the association with conditions that predispose to aspiration and infection in the gingival crevice, putrid discharge, and a high frequency of suppurative complications in late-stage disease.
ASPIRATION SYNDROMES
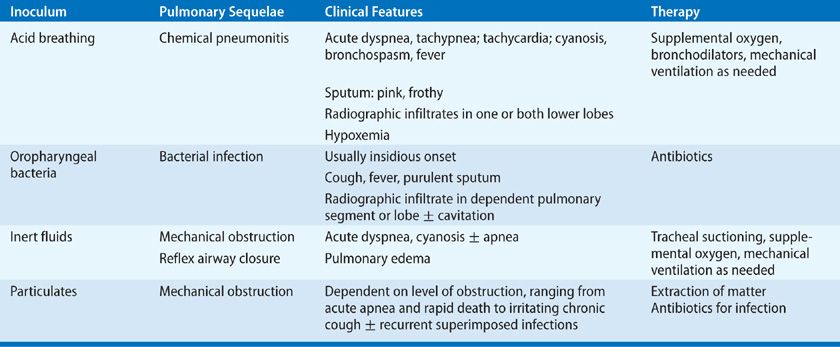
Aspiration is a relatively common event that is typically well tolerated. Numerous studies indicate that virtually all healthy persons aspirate, but that this is usually inconsequential. In one study, airway protective mechanisms were challenged by placing contrast material in the mouths of sleeping patients. The following morning, most patients had radiographic evidence of aspiration, defined as contrast material seen on imaging of the lungs, but no evidence of a disease process.32 Similarly, dye markers placed in the stomach of postoperative patients has later been aspirated from the tracheobronchial tree, indicating aspiration of gastric contents during general anesthesia in 7% to 16%.33,34 Scintigraphic methods have also been used to demonstrate frequent aspiration in patients with intubation of the airways or gastrointestinal tract.35,36 None of these studies, however, have demonstrated any clinical consequences from occult aspiration. The conclusion is that aspiration is relatively common but usually resolves spontaneously. The decisive factor for the development of lung complications depends on the frequency, volume, and character of the material in the inoculum. The aspiration of large volume or frequent aspirations lead to three distinct syndromes according to the nature of inoculum: chemical pneumonitis, bacterial infection, and airway obstruction. The nature of inoculum also dictates the pathogenesis of pulmonary complications, clinical presentation, and management strategies (Table 127-4).
 CHEMICAL PNEUMONITIS
CHEMICAL PNEUMONITIS
Chemical pneumonitis refers to aspiration of an inoculum that is inherently toxic to the lungs. Examples include acid, animal fats such as milk and mineral oil, and volatile hydrocarbons. These substances are toxic to the lower airways and initiate an inflammatory reaction. The effect of acid on the lungs was initially described in animal studies in World War I and subsequently confirmed in humans by Mendelson. His work was the first to describe gastric acid pneumonitis leading to chemical pneumonitis in humans, which came to be referred to as Mendelson’s syndrome.37 This is a severe pneumonitis with fever, hypoxia and respiratory alkalosis, which typically clears rapidly in healthy hosts within 4 to 7 days but may progress following lung injury and superinfection to pneumonia, lung abscess, or acute respiratory distress syndrome (ARDS) (see Chapters 140 and 141). Factors that contribute to hypoxemia are pulmonary edema, reduced surfactant activity, reflex airway closure, hyaline membrane formation, and alveolar hemorrhage. Pulmonary function tests show decreased compliance, abnormal ventilation–perfusion, and reduced diffusing capacity.2 The pathophysiology of gastric acid pneumonitis has been studied in experimental animals with intratracheal instillation of graded acid inocula. This work shows that the pH must be 2.5 or less, and a relatively large inoculum, usually a minimum of 0.3 to 0.6 mL/kg, is required for the inflammatory process to be initiated.38–41 It is possible that smaller volumes initiate a less dramatic presentation or may go undetected.39,41 Support for this hypothesis is the observation of frequent bouts of pneumonitis and otherwise unexplained pulmonary fibrosis in patients with gastric reflux or esophageal disease.42–44
Pathologic changes in acid pneumonia occur rapidly. Atelectasis occurs within seconds and is extensive within minutes. There is also peribronchial hemorrhage, pulmonary edema, and bronchial epithelial cell degeneration. The alveolar spaces fill with neutrophils within hours and hyaline membranes are seen within 48 hours. Resolution begins by the third day and may be complete or may result in residual scarring of the pulmonary parenchyma.45,46 Long-term follow-up studies in patients who have gastric acid pneumonia show either complete recovery or radiographic evidence of pulmonary fibrosis with abnormal gas exchange.47,48 Pulmonary fibrosis may also be associated with repeated, small, “silent” aspirations of gastric secretions without clinically overt pneumonitis (see Chapters 56 and 57).42,49
The diagnosis of acid pneumonia is usually presumed on the basis of clinical observations such as the abrupt onset of dyspnea in a patient who is aspiration prone and has radiographic evidence of infiltrates, usually in the lower lobes. Other characteristic clinical features are the rapid clearing of the infiltrates and progression to ARDS.46,50 Bronchoscopy demonstrates erythema of the bronchi, suggesting a “chemical burn.”51 Confirmation of the acid inoculum is not possible because of rapid neutralization by pulmonary edema fluid and bronchial secretions within minutes after aspiration.
The treatment of gastric acid aspiration includes tracheal suction to clear fluids and particulate matter that may have been concurrently aspirated. Supportive care consists primarily of ventilatory support with positive pressure ventilation and resuscitation with intravenous fluids.46,52 While use of corticosteroids dates back to the 1950s, clinical trials have not shown them to be beneficial.53–55 Antimicrobial agents are reserved for superinfection.
 ASPIRATION PNEUMONIA
ASPIRATION PNEUMONIA
While not as fulminant as chemical pneumonitis,56 bacterial infection following aspiration is associated with an increased risk of prolonged hospitalization and death.57,58 In the preantibiotic era, the natural history of these infections could be followed. The initial presentation was similar to chemical pneumonitis, usually combined with purulent sputum. After 8 to 14 days, progression to tissue necrosis with abscess formation or extension into the pleural space may occur. This stage is marked by putrid discharge and cavitation.59–61 The distinction between aspiration pneumonia and other forms of pneumonia is largely based on the clinical characteristics, but clear overlaps exist. For example, healthy elderly patients with community-acquired pneumonia have a markedly higher incidence of silent aspiration than matched control patients.62 While no gold standard for the diagnosis of aspiration exists, studies suggest that 5% to 15% of community-acquired pneumonia may be due to aspiration.11,63,64 The incidence of aspiration pneumonia in elderly patients with community-acquired pneumonia may be much higher.65,66 The diagnosis of aspiration pneumonia is often inferred from patient characteristics. Antimicrobials with specific anaerobic activity may only be indicated in patients with periodontal disease, patients expectorating putrid sputum, and patients with necrotizing pneumonia or lung abscess on chest radiograph.46,67–69
 MECHANICAL OBSTRUCTION
MECHANICAL OBSTRUCTION
Aspiration may involve fluid or particulate material. In this form of aspiration pneumonia, the inoculum is not toxic to the lung but may cause obstruction or reflux airway closure. In most cases there is only transient, self-limited hypoxemia due to rapid clearance.45 Some patients develop pulmonary edema, with hypoxemia and reduced compliance apparently due to an intrinsic pulmonary reflex closure.45 Other patients suffer sequelae due to failure to clear relatively large volumes of the aspirate, such as near-drowning victims and patients with profound neurologic deficits or in coma. The obvious critical intervention is tracheal suction.
Aspiration with mechanical obstruction may also be associated with solid particles. Foreign-body aspiration is most frequent in children 1 to 3 years of age.70–72 The most common objects in the lower airways are vegetable particles, inorganic materials, and teeth.70–72 The severity of the obstruction depends on the relative size of the material aspirated and the caliber of the lower airways. Large objects may cause obstruction at the level of the larynx or trachea, leading to sudden respiratory distress, cyanosis, stridor, and in some cases, aphonia.73,74 This is referred to as café coronary syndrome because it often involves meat aspiration during restaurant dining and may simulate an acute myocardial infarction.75 Aspiration of smaller particles may result in complete obstruction of more distant components of the tracheobronchial tree or partial obstruction. Chest radiographs often show atelectasis or obstructive emphysema.76 Common symptoms include chronic cough, fever, hemoptysis, and dyspnea.77,78 An important clue in some cases is unilateral wheezing.79 Bacterial infection is not important in the early stages of obstruction but is a common feature when obstruction has been present for more than 1 week.80 The most common pathogens are anaerobic bacteria from the upper airways. These patients may respond well to antibiotics, but often have recurrent infections in the same pulmonary segment.70 The most important therapeutic intervention is removal of the foreign body, usually with bronchoscopy.71
 RADIOLOGIC DIAGNOSIS
RADIOLOGIC DIAGNOSIS
Chest radiographs in patients with anaerobic lung infections typically demonstrate infiltrates (with or without cavitation) involving dependent pulmonary segments.1,46 The favored locations are the superior segment of the lower lobes or posterior segments of the upper lobes; these are dependent in the recumbent position. The basilar segments of the lower lobes are favored in patients who aspirate in the upright position. The right lung is more frequently affected, owing to the more direct takeoff of the right mainstem bronchus.
 LABORATORY DIAGNOSIS
LABORATORY DIAGNOSIS
Aspiration pneumonitis is a clinical diagnosis. It is generally assumed that there is an anaerobic component to pneumonia in patients with altered consciousness or after surgical procedures. It is important to emphasize the utility of the Gram stain in making the diagnosis of anaerobic lung infection. Often culture data are not available to confirm the presence of these organisms. However, most anaerobic gram-negative bacteria have unique morphologic features that make them relatively easy to identify or suspect on direct Gram stain. For example, peptostreptococci appear like their aerobic counterparts. Aspiration pneumonia is usually a mixed infection involving multiple bacteria; about half of the cases demonstrate mixtures of aerobic and anaerobic bacteria.1,81,82 Thus, the detection of polymicrobial flora or bacteria with the unique morphology of anaerobes on any specimen that is devoid of contamination by normal flora represents an important clue to the probable presence of anaerobic infection.
Determination of the microbiology of anaerobic infections of the lower airways requires a specimen devoid of contamination by the flora of the upper airways or quantitative cultures that distinguish pathogens from normal flora. Uncontaminated specimens that are considered valid for anaerobic culture include pleural fluid, TTAs, transthoracic aspirates, and specimens obtained at thoracotomy or thoracoscopy. Quantitative cultures of specimens obtained at fiberoptic bronchoscopy, either by BAL or with the protected brush, may also be used for this purpose.1 Anaerobic bacteriology should not be obtained from standard bronchoscopic aspirates. Quantitative culture of lower airway secretions improves diagnostic accuracy with virtually any specimen that is subject to contamination, including expectorated sputum and tracheostomy aspirates. Most studies employing these techniques have used them to detect aerobic bacteria; relatively few studies evaluated anaerobic cultures. Whether treatment decisions based on quantitative cultures of anaerobic pathogens improve treatment outcomes has not determined.
When possible, respiratory samples should be obtained before the inception of antibiotic treatment. In clinical practice, specimens are often not obtained for anaerobic culture until the patient has developed complications of persistent infection, which greatly reduces their yield. Thus, the anaerobic pathogens should be considered in the appropriate clinical context, even when only aerobic organisms are isolated.
It is essential that material obtained for culture be placed under anaerobic conditions promptly before transport to the laboratory. A sealed syringe provides the best container, with delivery of the specimen to the laboratory within 20 to 30 minutes for immediate plating. It is imperative that air bubbles be eliminated from the syringe and needle. Special anaerobic transport tubes are also available for brush or liquid specimens. It is important to obtain additional pulmonary specimens for culture and antibiotic susceptibility measurements from patients failing to respond to initial therapy (Fig. 127-2). Such data may demonstrate the presence of unrecognized or antibiotic-resistant organisms. Most of these infections are polymicrobial and many of the organisms grow slowly in vitro. Thus, it often takes several days to separate, identify, and report results of anaerobic cultures. There is great variation in the availability and quality of in vitro susceptibility tests. These factors illustrate the need for empiric decisions regarding antibiotic selection.
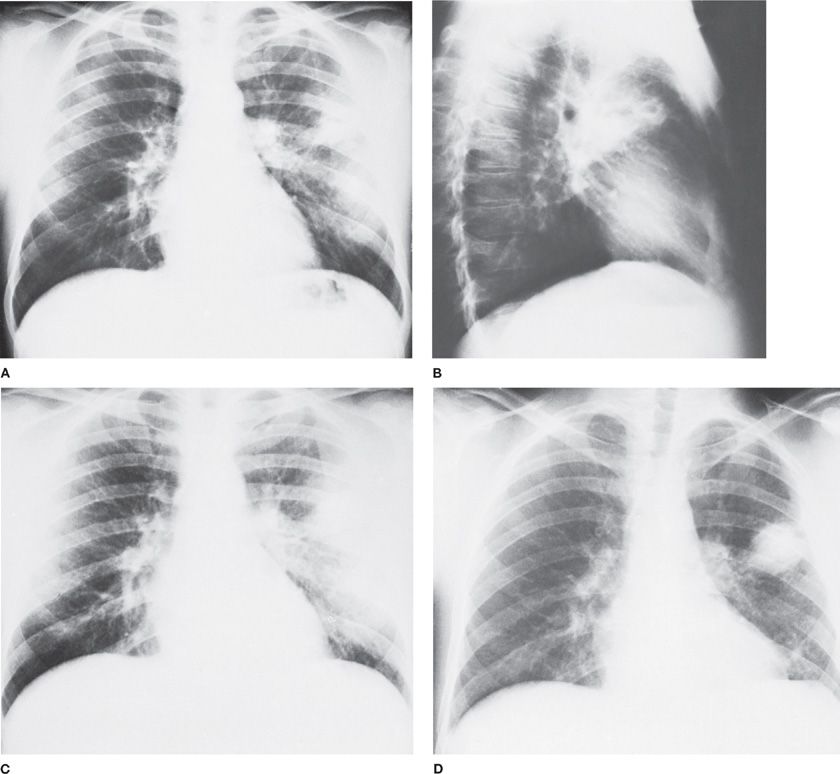
Figure 127-2 Failure of penicillin therapy for anaerobic lung abscess in a 29-year-old alcoholic man. A. Admission chest radiograph reveals a radiolucent area within a zone of consolidation in the left upper lung field. B. Lateral view demonstrates multiple cavities. The patient was treated for 5 days with penicillin (6 million units per day intravenously), followed by the same dosage orally for 10 days. C. Radiographic infiltrate persists but no cavity is visible. D. Six weeks after the cessation of penicillin therapy, the abscess has recurred in the same area. Marked pleural reaction is noted in the vicinity of the recurrent disease.
 PREVENTION
PREVENTION
The reversibility of underlying conditions predisposing to aspiration must be considered. It has recently been recognized, for example, that aspiration in lung transplant recipients is a major predisposing factor to graft injury and obliterative bronchiolitis.83 In the general patient population, nasogastric-feeding tubes, sedation, lying flat in bed while sleeping, reflux, and frequent choking are associated with aspiration and should suggest strategies for remediation.46 Gastric surgery for obesity has also been associated with a high incidence of aspiration disease.84
Positioning, dietary changes, medications, oral hygiene, and tube feeding, among other interventions, have been proposed to prevent aspiration both in hospitalized and nonhospitalized patients, particularly in the elderly. However, studies have failed to support a specific intervention in the outpatient setting.85 While using nectar or honey-thickened liquids decreases aspiration among patients with dementia or Parkinson disease,86 the incidence of pneumonia is not changed.87
Methods to prevent aspiration have been studied most extensively in hospitalized patients, especially in those who are aspiration prone. Most important is use of semirecumbent or upright positioning.88 Additional measures to prevent aspiration with variable efficacy include tracheostomy, reducing gastric contents by suction or metoclopramide, feeding via gastrostomy tube, and neutralization of gastric acid. Many of these procedures may alter colonization patterns and potentially may predispose to more significant infections. Neutralization of gastric acid may increase colonization of the oropharynx and thereby increase the risk of bacterial infection following aspiration of gastric contents. Tracheostomy is useful in some patients with repeated aspiration, but inflation of the balloon may occlude the esophagus and promote aspiration of upper airway contents. Patients who require nasogastric feedings are aspiration prone; percutaneous endoscopic gastroscopy (PEG) may be alternative method to address this issue, but study results are quite variable. A recent meta-analysis of nine studies found that while PEG feeding was superior to nasogastric tube feeding in providing consistent nutritional support, the incidence of pneumonia was no different between the two groups.89 Alternative procedures sometimes considered to prevent or limit aspiration include is postpyloric feeding jejunostomy and Nissen fundoplication.
 ANTIMICROBIAL THERAPY
ANTIMICROBIAL THERAPY
It is essential, whenever possible, to obtain microbiologic samples from the lungs and blood in advance of antimicrobial therapy. As for all pneumonias, inappropriate initial therapy has an adverse impact on outcome. The initial choice of antimicrobial agents should be guided by the Gram stain and the likely bacteriology of the infection and then adjusted as culture data become available. The history and a review of previous culture results may be useful in the selection of specific antibiotics. The standard drug historically for aspiration pneumonia and lung abscess involving anaerobic bacteria has been penicillin, usually given intravenously or with high-dose oral treatment. However, in the face of increasing penicillin resistance among S. pneumoniae and in 40% to 60% of strains of fusobacteria and P. melaninogenica as well as anaerobic gram-negative bacilli, alternatives should be considered for empiric therapy. In early therapeutic trials in patients with lung abscess involving anaerobic bacteria (Table 127-5), clindamycin proved superior to penicillin in terms of response rates and time to defervescence. Alternative regimens that have been used successfully based on anecdotal experience include amoxicillin–clavulanate and penicillin combined with metronidazole. Metronidazole should not be used as a single agent in patients with anaerobic lung infections, since there is a poor response in about 50%.90,91 The presumed explanation is the contributing role of aerobic and microaerophilic streptococci, which are resistant to this drug.