Occupational Disorders |
INTRODUCTION
Asbestos is a fibrous hydrated magnesium silicate with commercial use due to its indestructible nature, fire resistance, and easy transformability into industrial products from yarn to insulation block to brakes. Asbestos fibers are generally defined as long, thin fibers with a length to width ratio (aspect ratio) of 3:1. There are six commercial forms of asbestos: chrysotile, crocidolite, amosite, anthophyllite, actinolite, and tremolite. Most of the asbestos used in the United States has been chrysotile, a serpentine form of asbestos. Other asbestos types are the amphiboles—notably amosite, mined in South Africa, and crocidolite, mined in the Cape Province of South Africa and in Western Australia. These asbestos fiber types have strikingly different physical characteristics: chrysotile tends to be wavy and long, and occurs in bundles; crocidolite is needle-shaped with many long fibers; and amosite is similar to crocidolite but generally thicker. Asbestos fibers accumulate in the interstitium of the lung and are coated by iron and hemosiderin in a beaded, clubbed fashion referred to as ferruginous or asbestos bodies.
Initially, asbestos was widely used in fireproof textiles and later as insulation for boilers and pipes. Thereafter, asbestos was used in yarn, felt, paper, millboard, shingles, paints, cloth, tape, filters, wire insulation, cement pipes for potable water, gaskets, and in friction materials, including brake linings, and roofing and floor products. Asbestos was extensively used for ship construction during World War II. Occupational exposure to asbestos in the United States now primarily occurs during maintenance activities or remediation of buildings containing asbestos. The Occupational Safety and Health Administration (OSHA) has estimated that 1.3 million workers in general industry continue to be exposed to asbestos, and the National Institute for Occupational Safety and Health (NIOSH) estimates that 44,000 mine workers might be exposed to asbestos fibers or amphibole cleavage fragments, especially tremolite. Although the European Union has banned imports and use of asbestos, approximately 2 million tons are used worldwide. Asbestos is mined in Eastern Europe and Asia where asbestos products are manufactured for use in insulation, roofing, and construction. Globalization has resulted in export of asbestos to developing countries for manufacture and export to other developing countries with concomitant increase in asbestosis and mesothelioma.1
TYPES OF EXPOSURE
Primary asbestos exposures occurred in miners and millers. Secondary exposures occurred in manufacturing plants using asbestos in the production of textiles, friction materials, tiles, and insulation materials. Epidemiological studies focused on cohorts in these plants, since asbestos fiber type was often specified and dust measurements were obtained. These studies demonstrated that intensity and duration of exposure play an important role in the prevalence of asbestos-related disease. Common trades with asbestos exposure include sheet metal work, plumbing, pipefitting, insulation, railroad and utility work, and school or building custodians. Prevalence of radiographic opacities in asbestos insulation workers exposed prior to the 1970s reached 50% in long-term workers and was approximately 15% in sheet metal workers. There is a long latency period between exposure and manifestation of asbestos-related disease making identification and intervention difficult.
Although measurements of airborne asbestos fibers were seldom made, the most significant exposures appear to have occurred in the construction trades. These trades included asbestos insulators (called “laggers” in the United Kingdom), who mixed asbestos cement on site to insulate joints and elbows on pipes; boilermakers and sheet metal workers, who worked adjacent to the asbestos workers; and electricians, carpenters, plumbers, and others who worked in the vicinity of work requiring asbestos exposure. These exposures were mainly to chrysotile asbestos, since practically no crocidolite was imported into the United States, and only small amounts of amosite were admixed. Asbestos workers and other construction workers wore their asbestos-covered clothes home, exposing their wives and children when greeting them or while washing their garments. These household contact exposures are often referred to as indirect exposures, and those exposed while working near asbestos workers are called bystander exposures.
Projected lung cancer deaths range from 55,000 to 76,700 for the 30-year period due to asbestos exposure over 1980 to 2009. The National Occupational Respiratory Mortality System has tracked asbestosis deaths since 1968, peaking at 1400/y since 2004 and mesothelioma deaths peaking at 2700/y since 2005. Asbestosis is the cause of about 11% of deaths in asbestos workers; interestingly, the most important determinant is radiographic profusion score with rates increasing from 2.4% to 10.98% and 35.4% with profusion category increasing from 1 to 3. Profusion of irregular opacities is categorized into four levels ranging from 0 to 3, with 12 subcategories 0/0 to 0/1 to 1/0 up to 3/4 for the most dense fibrosis according to a scale agreed to by the International Labor Organization. Dyspnea, a low FVC, and/or physical examination findings typical of interstitial fibrosis (rales, clubbing or cyanosis) raise the risk of death from asbestosis from two- to sixfold.2
NONMALIGNANT PLEURAL MANIFESTATIONS
Pleural disease is the most common manifestation of asbestos exposure. The nonmalignant manifestations of asbestos exposure in the pleural space include circumscribed pleural plaques, diffuse pleural thickening, rounded atelectasis, and asbestos-related pleural effusions.3
 PLEURAL PLAQUES
PLEURAL PLAQUES
Important pathogenetic and clinical features of pleural plaques are discussed below.
Pathology
Pleural plaques are the most common manifestation of asbestos exposure. They are focal, irregular, raised white lesions found on the parietal and, rarely, the visceral pleura. The plaques may be small or extensive; commonly they occur in the lateral and posterior midlung zones, where they may follow rib contours and the diaphragm. They commonly enter lobar fissures and can invade the mediastinum or pericardium; rarely do they invade the apices or costophrenic sulci. Histologically, asbestos-related pleural plaques are characterized by a paucity of cells, extensive collagen fibrils arranged in a basket-weave pattern, and a thin covering of mesothelial cells. The parietal pleura is uniformly involved, with minimal thickening of the visceral pleura. The two pleural surfaces are free of adhesions. Pleural calcifications frequently develop in these fibrohyaline lesions as the length of time from exposure increases. Exposure to asbestos is the most frequent cause of pleural plaques.
Pathogenesis
Two theories have been proposed for the pathogenesis of pleural plaques. The most plausible is based on the direct effects of fibers that reach the pleural space. Asbestos fibers – the short, thin ones in particular – have been shown to be transported by subpleural lymphatics to the pleural space. In the pleural space, it is believed that they scratch, injure, and irritate the pleural surface, leading to hemorrhage, inflammation, and eventually fibrosis. The plaques are submesothelial. Mesothelial cells appear to play an important role in the pathogenesis of these lesions: they internalize asbestos fibers via an integrin receptor that recognizes vitronectin; in vitro pleural mesothelial cells also can synthesize collagens (types I, III, and IV), elastin, laminin, and fibronectin. In keeping with the submesothelial location of the plaques, cultured mesothelial cells can organize these macromolecular connective tissue components into an assemblage of extracellular matrix that is limited to the base of the cell.
Epidemiology and Natural History
Hyaline and calcified pleural plaques have been noted to be an index of exposure to asbestos. In shipyard workers, the frequency of pleural abnormalities was approximately 10 times that of parenchymal disease. The greater the exposure, the more likely the worker was to have extensive calcified pleural plaques as well as parenchymal fibrosis. The intensity of the exposure has been noted to be an important determinant of the prevalence of these abnormalities. For example, among British shipyard workers, 36% of those with continuous exposure as “laggers” developed pleural plaques, while extensive pleural thickening and pulmonary fibrosis were seen in 5% and 7%, respectively. In contrast, those with intermittent exposure had a 6% prevalence of plaques and no pulmonary fibrosis. On average, the latency time for the appearance of plaques is 30 years, but the time can vary greatly. This variation can also be appreciated from studies of British shipyard workers in whom the prevalence of pleural plaques increased from 17% at 10 years after the first exposure to 70% at 30 years among those with continuous exposure; for those with intermittent exposures, the prevalence increased from 1% at 10 years to 16% at 30 years.
All asbestos fibers are equally capable of inducing pleural plaques: Pleural plaques are found in US insulators or shipyard workers exposed to chrysotile or amosite, as well as miners in Western Australia who were exposed to crocidolite.
In addition to occupational exposures, environmental, domestic and residential exposures have been implicated in the production of pleural plaques. Evidence for the latter is the remarkably high rates of pleural calcification (up to 45%) in some rural areas of Greece, Corsica, Cyprus, and Turkey where outcroppings of tremolite asbestos have been used to whitewash houses. Pleural plaques have been noted from environmental exposure to tremolite asbestos fibers contaminating vermiculite mining and milling in Libby, Montana.
Clinical and Physiological Features
In the absence of concomitant asbestosis or obliteration of the costophrenic angle, pleural plaques are usually asymptomatic. Most often they are incidental findings on chest radiographs. In addition, they do not cause significant abnormalities such as pleural rubs, rales, or rhonchi on auscultation of the chest.
Pleural disease has been recognized as a cause of reduced pulmonary function since the 1970s. Among 998 shipyard workers in Groton, Connecticut, who had 15 or more years of asbestos exposure, 17% of those with pleural changes had a forced vital capacity (FVC) under 80% of predicted; for those with normal chest radiographs, 9% had decreased vital capacities. In those with normal chest radiographs, the values were significantly reduced only among smokers and ex-smokers. Recent studies that have applied stepwise regression analysis to data from insulation workers have disclosed a significant inverse relationship between FVC and an integrative pleural index for patients with circumscribed pleural plaques.4 Even among those with pleuroparenchymal abnormalities, the pleural index was found to make a significant contribution to decrements in FVC, independent of that due to parenchymal abnormalities.
In nonsmoking asbestos workers with circumscribed or diaphragmatic pleural plaques, flow rates (FEV1, FEF25–75%) have been reported to be reduced. In an epidemiological study of 1211 sheet metal workers, pleural fibrosis was detected in 334 and was related to age, duration of exposure, more pack-years of smoking, and the presence and degree of interstitial fibrosis. After controlling for these confounders, multivariate regression analysis found that both plaques and diffuse thickening were independently associated with decrements in FVC, but not with decrements in the FEV1/FVC ratio. Furthermore, diffuse pleural thickening was associated with a decrement in FVC twice as great as that seen with circumscribed pleural plaques. After confounding variables such as age, height, smoking status, and the presence of parenchymal abnormality as assessed by chest radiography and gallium scintigraphy were taken into account, there was a significant decrease in FEV1 and FVC (222 and 402 mL, respectively) among workers who had pleural plaques or diffuse pleural fibrosis.
Radiographic Features
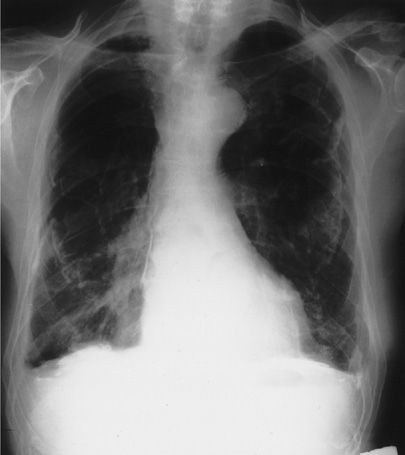
The visualization of plaques on digital chest radiography depends on their thickness, location, and the orientation of the radiographic beam.5 As a result, they can be viewed in profile along the lateral chest wall or on en face with a rolled or holly-leaf pattern, especially if calcified (Fig. 86-1). Only a modest proportion of plaques detected at autopsy can be seen on digital chest radiography. Computed tomographic (CT) scanning increases plaque detection (increase in sensitivity and specificity).
Figure 86-1 Posteroanterior (PA) chest radiograph of a 75-year-old man who worked in a shipyard during World War II insulating ships. The radiograph shows bilateral calcified pleural plaques en face and on top of the diaphragm. The pleura is diffusely thickened bilaterally and the costophrenic angles are blunted. Mediastinal pleural calcification is present on the right. (Used with permission of Dr. Timothy Harkin.)
The CT scan can recognize plaques at a much earlier and less well-defined state than digital chest radiography. The CT scan is particularly useful for perivertebral and pericardiac plaques, and high-resolution CT scanning (HRCT) helps to establish the presence of diaphragmatic lesions. In all cases, the CT scan can help to differentiate plaques from extrapleural fat pads and can detect concomitant parenchymal abnormalities that may be difficult or impossible to see on the PA chest radiograph.
Diagnosis
Pleural plaques due to asbestos exposure are usually bilateral (80% of the time), whereas unilateral pleural plaques may be due to trauma, previous tuberculosis, or, rarely, other causes, such as collagen vascular disease. The lesions are usually stable and remain the same size for months. This helps to differentiate plaques from pleural tumors. Histological tissue examination is not necessary for diagnosis the vast majority of the time.
Treatment
No specific treatment is required for asbestos pleural plaques. Since they are markers of asbestos exposure and identify patients at risk for other asbestos-related disorders, medical surveillance, including periodic CT scans and blood biomarkers, are recommended.6
 DIFFUSE PLEURAL THICKENING
DIFFUSE PLEURAL THICKENING
Pleural thickening, another common manifestation of asbestos exposure, is discussed below.
Pathology
Pleural fibrosis in persons who have been exposed to asbestos has been well described. The fibrotic responses can be localized or diffuse and either unilateral or bilateral. Macroscopically, the lesions vary in thickness from a whitish discoloration of the lung surface to a thick white peel that can encase significant pulmonary structures. Diffuse pleural thickening is most often seen as a continuous sheet that is 5 to 10 cm in craniocaudal extent, and in 90% of patients it affects the costophrenic angle. Interlobar and interlobular fissures are commonly involved. Whereas pleural plaques predominantly affect the parietal pleura, diffuse pleural fibrosis occurs most commonly as part of a fibrotic process of the visceral pleura and subadjacent interstitium.
Pathogenesis
Diffuse pleural thickening has been proposed to result from three different mechanisms. The first is the confluence of large pleural plaques. This is believed to account for 10% to 20% of the cases. The second is the extension of subpleural fibrosis to the visceral pleura. This probably accounts for 10% to 30% of cases. The most common pathogenic mechanism is thought to be the fibrotic resolution of a benign pleural effusion, producing diffuse pleural thickening. The importance of this mechanism is highlighted by the finding that about one-third of patients with diffuse pleural thickening have had a prior benign asbestos-related pleural effusion diagnosed by thoracentesis or on serial chest radiographs. The pathogenic mechanisms differentiating diffuse pleural thickening from circumscribed pleural plaques are not well defined. However, the fundamental mechanism of asbestos fibers activating macrophages and mesothelial cells to release growth factors and stimulate collagen formation is likely to be important in both. In the case of diffuse pleural responses, these fibers are deposited mainly in the parenchymal subpleural areas of the lung.
Clinical and Physiological Manifestations
Diffuse pleural fibrosis most often occurs long after short-term heavy exposure to asbestos. When mild, diffuse pleural fibrosis can be asymptomatic and discovered as an incidental finding on a chest radiograph obtained for another reason. The diffuse nature of the lesion, however, often leads to pulmonary symptoms, including dyspnea on exertion, chronic dry cough, and chest pain. As noted earlier, diffuse pleural thickening can cause a restrictive physiological abnormality. The degree of physiological abnormality varies with the degree of fibrotic response. On rare occasions, in patients with severe bilateral disease, respiratory insufficiency has occurred. Diffuse pleural fibrosis can increase in severity over time.
Radiographic Features
On the routine chest radiograph, diffuse pleural fibrosis presents as a continuous pleural opacity extending over more than 25% of the pleural surface of a lung, often blunting the costophrenic angle. It can be unilateral or bilateral and seen in the presence or absence of concomitant asbestosis and pleural calcifications. Rarely, the pleural fibrosis will produce a fibrotic pseudotumor with a pleural basis (rounded atelectasis) (see below). CT scanning is particularly useful in delineating the relationship between diffuse fibrosis and other pleural abnormalities and differentiating pleural fibrosis from fat deposits.
Diagnosis
The diagnosis of diffuse pleural fibrosis is usually based on the clinical presentation and chest radiograph. The lesions of diffuse pleural fibrosis are not unique to asbestos-exposed persons and can represent old inflammatory reactions from tuberculosis, thoracic surgery, or hemorrhagic chest trauma. Differentiation among these causes is frequently based on a careful clinical history. Radiographic patterns are also helpful, since bilateral interstitial changes in the lower lung zones in association with pleural plaques or calcifications strongly support a diagnosis of asbestos exposure. A biopsy may be required when the thoracic lesion is progressing or when malignancy is in the differential.
Treatment
As seen with circumscribed pleural plaques, there are no specific therapies for asbestos-related diffuse pleural fibrosis. Medical surveillance is required to detect disease progression, lung cancer, and mesothelioma.
 ROUNDED ATELECTASIS
ROUNDED ATELECTASIS
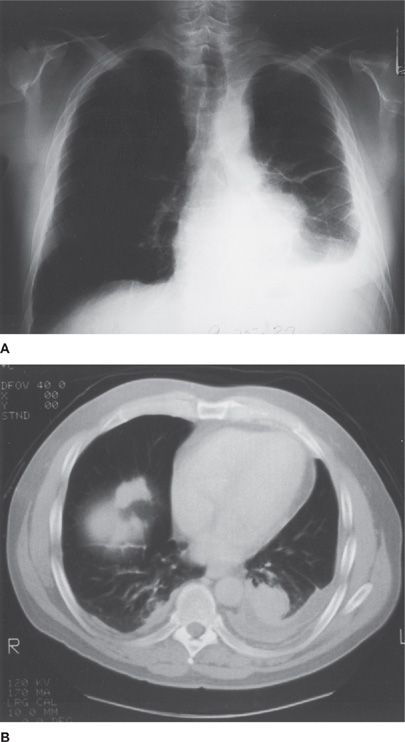
Rounded atelectasis is a rare complication of asbestos-induced pleural disease. It is caused by scarring of the visceral and pari etal pleura and the adjacent lung, with the pleural reaction folding over on itself. The pleural surfaces then fuse to one another, trapping the underlying lung and leading to atelectasis. As a result of this alteration, a mass lesion that mimics lung cancer can be seen on the PA chest radiograph (Fig. 86-2). This lesion is most easily appreciated to be a pseudotumor with use of CT scanning. HRCT can noninvasively demonstrate continuity to areas of diffuse pleural thickening, evidence of volume loss in the adjacent lung, or a characteristic comet tail of vessels and bronchi sweeping into a wedge-shaped mass (Fig. 86-2).
Figure 86-2 Rounded atelectasis and other pleural abnormalities in an asbestos worker. The chest radiograph (A) shows a left-sided pleural effusion, bilateral pleural thickening, greater on the left than on the right, and a mass in the left midlung field. HRCT (B) demonstrates the mass to be rounded atelectasis, with bronchovascular structures entering the trapped lung. It also reveals the pleural effusion, bilateral pleural thickening, and pleural plaques, one of which is on the right hemidiaphragm. (Used with permission of William M. Rom, MD, MPH.)
CT scanning can also demonstrate stability over time (from months to years), which supports the diagnosis of a benign lesion, and pleural plaques or parenchymal changes, which support a diagnosis of asbestos exposure. In one clinical series of 74 patients with rounded atelectasis, 64 had significant asbestos exposure, and the lingula or right middle lobe was affected in 49 of the patients.7 HRCT scans localized most cases of rounded atelectasis to the lower, posterior portion of the lung; moreover, in one-third of the patients, the lesions were multiple. In most patients, rounded atelectasis occurs suddenly on a background of only plaques or a normal chest radiograph. In others, a slowly increasing pleural effusion may precede its appearance.
 ACUTE BENIGN PLEURAL EFFUSIONS
ACUTE BENIGN PLEURAL EFFUSIONS
Acute benign pleural effusions are common pleural manifestations in asbestos-exposed persons between 20 and 40 years of age. The latency period for these effusions is shorter than for pleural plaques, malignant mesotheliomas, or pulmonary malignancies. Benign pleural effusions generally occur earlier after exposure than do other asbestos-related processes; the latency is shorter, for example, 12 to 15 years rather than >20 years after the first asbestos exposure compared to pleural plaques or asbestosis.
About 50% of the patients with acute benign pleural effusions are asymptomatic. When patients are symptomatic, the manifestations may be those of a pleurisy (chest pain, chest tightness, dyspnea, cough, and fever). Physical examination reveals the signs of a pleural effusion; a pleural friction rub may be heard. The effusions are exudative and often bloody; glucose concentrations are normal. Mesothelial cells in effusions are found in about 50% of patients. In about 25% of patients, the fluid is eosinophilic. Rarely are asbestos bodies found even though they may be present in underlying lung tissue.
The designation “benign” refers to the lack of evidence of malignancy. The collections may persist for 6 months or more. They frequently clear spontaneously, only to recur on the contralateral side. However, a benign asbestos pleural effusion is a risk factor for the development of pleural thickening, especially diffuse pleural fibrosis. The diagnosis of acute benign pleural effusions is one of exclusion. Thoracentesis is essential. Pleural biopsy is frequently required to rule out other causes of pleural effusions, including mesothelioma. The usual pathological findings are a chronic fibrous pleurisy with minimal cellularity. Diffuse malignant pleural mesothelioma invariably presents as a pleural effusion, but pleural mass lesions are usually seen on CT scan, which are absent in benign asbestos effusions.
ASBESTOSIS
Important pathologic and clinical aspects of asbestosis are discussed below.
 PATHOLOGY
PATHOLOGY
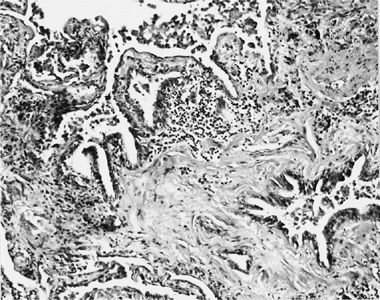
Asbestosis is the interstitial pneumonitis and fibrosis caused by exposure to asbestos fibers.3 Early lesions are characterized by discrete areas of fibrosis in the walls of respiratory bronchioles. The septi adjacent to the respiratory bronchioles are often thickened, and the fibrosis sometimes appears to spread outward from the bronchioles. In addition to the peribronchiolar fibrosis, there is an intense peribronchiolar cellular reaction that may narrow and obstruct the airway lumen. Macrophage accumulation is a prominent feature of this cellularity. Proliferation of type II alveolar epithelial cells is enhanced. The interstitium may contain collections of lymphocytes; smooth muscle proliferation may be prominent in areas of remodeling; and buds of loose connective tissue may be seen within the alveoli (Fig. 86-3).8 Initially, the disease usually involves first-order bronchioles; subsequently, second- and third-order bronchioles are affected. As the disease progresses, the fibrosis becomes diffuse, the architecture of the lung undergoes extensive remodeling, and honeycombing supervenes. In contrast to other pneumoconioses, lymph node enlargement and progressive massive fibrosis do not occur. Pathologically, the alterations seen in asbestosis cannot be differentiated from many other interstitial fibrotic disorders except for the presence of asbestos bodies and uncoated asbestos fibers.
Figure 86-3 Lung tissue from a 64-year-old asbestos insulator with 46 years of exposure to asbestos while insulating pipes. His chest radiograph revealed extensive irregular opacities and bilateral pleural thickening. The figure illustrates peribronchiolar fibrosis, interstitial chronic inflammation, accumulation of macrophages in the airspaces, and proliferation of type II pneumocytes. (Reproduced with permission from Rom WN, Travis WD, Brody AR. Cellular and molecular basis of the asbestos-related diseases: State of the art. Am Rev Respir Dis.1991;143(2):408–422.)
 PATHOGENESIS
PATHOGENESIS
Asbestos fibers are deposited at airway bifurcations and in respiratory bronchioles and alveoli by impaction, sedimentation, and interception. Fibers then migrate into the interstitium, in part via an uptake process involving type I alveolar epithelial cells. This causes alveolar macrophages to accumulate in the alveolar ducts, peribronchiolar interstitium, and alveolar spaces, constituting an alveolar macrophage alveolitis. Following this initial macrophage alveolitis, most fibers are cleared, leaving the lungs unscarred. If clearance is incomplete, fibrosis can ensue. The degree of fibrosis in asbestosis relates, in general, to the lung dust burden. If the dust load is small, the tissue reaction may be limited and the disease may be mild and not progress. If the retained dust load is great, tissue reaction and macrophage alveolitis are proportionately more intense, greater injury occurs, and chronic and progressive lung disease can develop.
The macrophage alveolitis that is seen in early stages of asbestosis results from monocyte recruitment from the blood and in situ macrophage replication. These cells appear to play an important role in the pathogenesis of the inflammation and fibrosis seen in this disorder. Morphologically, they express an activated phenotype characterized by cellular multinucleation and a striking increase in membrane ruffling, surface blebbing, and lysosomes and phagolysosomes.9 These macrophages are presumably attempting to engulf and clear the asbestos fibers. This process is not uniformly successful, however. First, the fibers induce apoptosis in the cells. Although the coating of asbestos fibers to form asbestos bodies makes them less toxic, the vast majority of fibers in the lung remain uncoated. Second, the long fibers cannot be completely phagocytosed. Finally, chrysotile asbestos fibers tend to split longitudinally. This generates additional fibers that can multiply the asbestos effect even after exposure has ceased. As a result, asbestos has a prolonged residence, surprising mobility, and penetrates the interstitium of the distal lung.
These characteristics probably contribute to the pathogenesis of the disease, since – in contrast to inert particles, which can be ingested by macrophages and cleared without generating a significant response – asbestos fibers stimulate macrophages to produce a variety of important cytokines and growth factors. Both asbestos and silica are phagocytosed by alveolar macrophages, which then activates them to release cytokines, growth factors, and oxidants. These include platelet-derived growth factor (PDGF), and insulin-like growth factor-1 (IGF-1), transforming growth factor-β, and cytokines interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and IL-8, the matrix molecule fibronectin, oxygen free radicals, and matrix metalloproteinases.10–12 The oxygen radicals contribute to tissue injury via direct cell cytotoxicity and lipid peroxidation of membrane components. The IL-8 recruits neutrophils to sites of disease activity. The PDGF, IGF-1, IL-1, TNF-α, and fibronectin contribute to tissue fibrosis by stimulating fibroblast proliferation and chemotaxis and collagen biosynthesis.8 In vitro exposure of mononuclear phagocytes to silica, asbestos, or coal activates transcription factors including AP-1 and NF-κB with subsequent release of cytokines and growth factors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree