Carotid artery stenting (CAS) was recently accepted as an alternative minimally invasive revascularization approach to carotid endarterectomy (CEA) for the treatment of high-risk patients with significant extracranial carotid artery stenosis and data in patients at normal risk for CEA is emerging. Although CEA is traditionally considered the “gold standard” therapy to prevent strokes in patients with carotid stenosis, this technique is invasive and may cause serious complications (death, stroke, myocardial infarction, cranial nerve palsy, wound infection, hemorrhage, etc). Consequently, carotid angioplasty and stenting was pursued almost three decades ago because it offered a less invasive approach that had the potential to be better tolerated with potentially fewer complications.
The idea of carotid angioplasty in humans was proposed in 1977 by Mathias (1), and subsequently performed by Kerber in 1980 (2). The early experience with carotid angioplasty was fraught with challenges and complications, attributed to lack of dedicated equipment, lack of protection from distal embolization, operator inexperience, and suboptimal patient selection. One of the earliest randomized carotid angioplasty trial (widely known as the “Stopped trial”) was aborted after enrolling 17 patients, since 5 of the 7 carotid angioplasty procedures resulted in strokes, compared to none in the CEA arm (3). These results were likely due to the combination of operator inexperience, poor patient and lesion selection, and crude instrumentation. Fortunately, further developments in technique and equipment by early interventional pioneers have dramatically reduced acute procedural complications and improved the long-term durability of the therapy.
Emboli protection devices (EPD) and self-expanding stents are key innovations that contributed to the success of CAS and represent the standard for contemporary CAS procedures. As of mid-2008, there are six FDA-approved carotid stent and EPD systems: the AccuLink™ stent and AccuNet™ filter (Abbott Vascular, Santa Clara, CA), the Xact® stent and EmboShield™ filter (Abbott Vascular, Santa Clara, CA), the Precise™ stent and AngioGuard XP™ filter (Cordis Corporation, Warren, NJ), the Protégé® stent and SPIDER™ filter (ev3 Inc., Plymouth, MN), the Wallstent and Filterwire EZ™ filter (Boston Scientific, Nattick, MA), and the Exponent® stent and GuardWire™ device (Medtronic, Minneapolis, MN).
Since the advent of carotid angioplasty and stenting, numerous studies have been executed to evaluate the procedural complications and long-term efficacy, especially in comparison to CEA. We will review these studies systematically, including (1) early large single-center and multicenter CAS registries, (2) contemporary pivotal high-risk CAS registries (several of which led to the FDA premarket approval (PMA) of six current stent and EPD devices), (3) randomized CAS versus CEA studies, (4) other comparative CAS versus CEA studies, (5) postmarketing registries, and (6) ongoing CAS trials.
EARLY CAS REGISTRIES
Several early single-center observational studies have been published. However, these studies included only small patient numbers, had short clinical follow-up, utilized different equipment (legacy stents largely without EPD), and employed inconsistent definitions of complications (e.g., minor or major strokes, ipsilateral or all strokes). Larger multicenter registries were thus performed, for example, the Pro-CAS, ELOCAS, and the Global Carotid Artery Stent Registry, which in aggregate included over 18,000 patients with variable surgical risks and provided valuable procedural and outcome data on CAS (4–6).
ELOCAS
The European Long-term Carotid Artery Stenting Registry included 2172 CAS procedures from 1993 to 2004 at four high-volume European centers in Belgium, Germany, and Italy (4). This registry included a mixture of prospective and retrospective cases, with inconsistent neurologic assessment and no independent assessment of clinical events. Technical success rate was 99.7%, stenting was performed in 95.6%, and EPDs were used in 85.9% of cases. Symptomatic patients represented 41.6% of the cohort. The 30-day death and major stroke event-rate was only 1.2%, with no significant difference between symptomatic (1.4%) and asymptomatic patients (1.0%). The long-term death and major stroke rates were 4.1%, 10.1%, and 15.5% at 1, 3, and 5 years, respectively. Restenosis rates were low, 1% at 1-year, 2% at 3-year, and 3.4% at 5-year follow-up. Interestingly, in a nonprespecified subgroup analysis, predilatation was associated with lower stroke and death event-rate compared with direct stenting (2.7% vs. 4.6% at 1 year, p = 0.002).
The Global Carotid Artery Stent Registry
The Global Carotid Artery Stent Registry commenced in 1997 and initially involved 24 centers in Europe, North America, South America, and Asia and had similar limitations with respect to inconsistent neurologic evaluation and event adjudication. By September 2002, there were 53 participating centers and 12,392 CAS procedures had been performed involving 11,243 patients, symptomatic patients representing 53.2% of the cohort (5). Technical success rate was 98.9%, and EPDs were used in 38.5%. The 30-day incidence of TIA was 3.1%, minor stroke 2.1%, major stroke 1.2%, and procedural death 0.6%. The combined 30-day stroke plus procedural death incidence progressively improved over the course of follow-up, from 5.7% to 4.0%, presumably related to equipment advancement, EPD use, and operator experience. In subgroup analyses, the 30-day stroke and procedural death was 4.9% in symptomatic patients and 3.0% in asymptomatic patients, and was 2.2% in patients who had EPD use (4221 cases) versus 5.3% in patients without EPD use (6753 cases). Both symptomatic and asymptomatic patients benefited from EPD use. In symptomatic patients, the 30-day stroke and death rate was 2.7% with EPD use versus 6.0% without EPD use. In asymptomatic patients, the 30-day stroke and death rate was 1.8% with EPD use versus 4.0% without EPD use. However, there was a steep learning curve for EPD use; centers that had performed 20 to 50 cases had a 4.0% stroke and death rate compared with 1.6% in centers that had performed >500 cases. At follow-up, ipsilateral neurologic events were observed in 1.2%, 1.3%, and 1.7%, at 1, 2, and 3 years, respectively. Restenosis rate by carotid ultrasound was low at 2.4% at 3 years.
Pro-CAS
The German Prospective Registry of Carotid Artery Angioplasty and Stenting (Pro-CAS) included 3267 CAS procedures performed between 1999 and 2003 at 38 centers in Germany, Austria, and Switzerland (6). Independent neurologic assessments were voluntary, inconsistent and not standardized, and clinical events were site reported only and not independently adjudicated. Stents were used in 98% of cases, of which 89% were self-expanding stents. The use of EPDs varied widely between the centers, and was recorded at 64% starting from October 2000 (not recorded prior). The overall technical success rate was 98%. The incidence of major stroke, minor stroke, and death was 1.2%, 1.3%, and 0.6%, respectively, and the combined incidence of stroke and death was 2.8%. Symptomatic patients represented 56% of the cohort. The incidence of stroke and death was 3.1% for symptomatic patients, and 2.4% for asymptomatic patients. There was no clear advantage with EPD use in this registry. In their most recent 2008 update encompassing 5341 CAS procedures performed between 1999 and 2005, the inhospital stroke and death event-rate was 3.6% (7). There was a progressively lower prevalence of procedural stroke and death event rates from the inception of the trial (6.1% during the first year of enrolment) to the later years (3.0% from 2004 to 2005). Several factors were found to predict periprocedural events: center experience, age, prior symptoms, primary intervention (vs. restenotic intervention), angioplasty (vs. stent), predilatation, and heparin dose >5000 units.
CONTEMPORARY HIGH-RISK CAS REGISTRIES
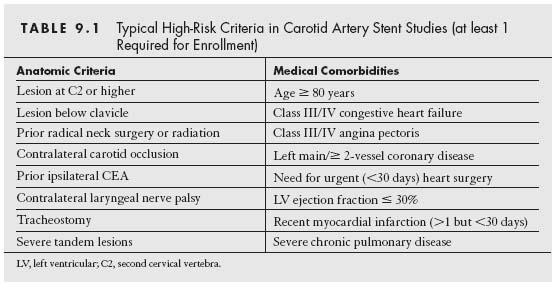
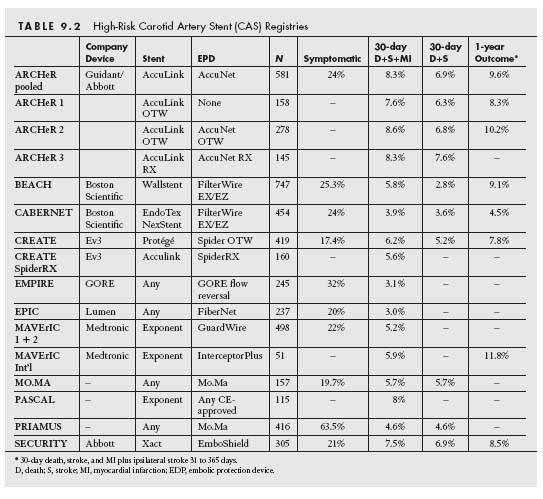
With the development of dedicated carotid self-expanding stents and EPDs, contemporary registries were initiated by the respective sponsors to evaluate the safety and efficacy of these systems in a highly regulated and prospective fashion in multicenter Investigational Device Exemption (IDE) trials. These contemporary registries specifically targeted patients at high-risk for surgical endarterectomy, since intuitively these patients would have the greatest benefit from a less invasive approach. These studies included both symptomatic and asymptomatic patients with carotid stenosis severity ≥50% and >80%, respectively. The high-risk inclusion criteria included a combination of high-risk anatomic factors and medical comorbidities (Table 9.1). These studies had independent neurologic assessments pre- and postprocedures, and were monitored by safety committees and adjudicated by independent clinical events committees (which was demonstrated to increase the rate of endpoint events by ~50%). The primary safety endpoints evaluated typically comprised a composite of death, stroke, and myocardial infarction (MI) at 30 days. The primary efficacy endpoint usually included the 30-day death, stroke, and MI events, combined with death and stroke events from 31 days to 1-year post-CAS. Overall, the 30-day composite death, stroke, or MI event-rate from these registries involving high surgical risk patients range from 3.0% to 8.3% (with 30-day death or stroke rates varying between 2.8% and 7.6%), and the 1-year event-rates were 4.5% to 9.6% (Table 9.2). The results from these IDE trials utilizing specific stent and EPD platforms were predominantly used to obtain CE Mark and FDA approval in Europe and the United States, respectively.
ARCHeR
The ARCHeR (AccuLink™ for Revascularization of Carotids in High-Risk patients) study consisted of three sequential, multicenter, nonrandomized prospective registries. The AccuLink™ over-the-wire (OTW) self-expanding nitinol stent was used without an EPD in ARCHeR 1, and with the AccuNet™ OTW filter EPD in ARCHeR 2 (both systems by Guidant Endovascular, now Abbott Vascular). ARCHeR 3 used the rapid-exchange versions of both stent and filter systems. Of 581 patients enrolled from 2000 to 2003 at 48 sites, 24% were symptomatic. Data from the three phases were similar, and thus the results were pooled and published in 2006 (8). The 30-day major adverse event-rate (MAE) of death/stroke/MI was 8.3% (13.1% in symptomatic patients, 6.8% in asymptomatic patients) and stroke/death rate was 6.9% (11.6% in symptomatic patients, 5.4% in asymptomatic patients). The majority of stroke events were minor (72%), with a major stroke rate of 1.5% (4.3% in symptomatic patients, 0.7% in asymptomatic patients). The primary efficacy endpoint (composite of 30-day MAE plus ipsilateral stroke between 31 days and 1 year) was 9.6%, which was noninferior to historical controls at 14.4%. Target lesion revascularization was 2.2% at 1 year and 2.9% at 2 years. These results led to FDA approval of the AccuLink™ stent and AccuNet™ EPD in August 2004, which was the first approved CAS device platform in the United States.
BEACH
The Boston Scientific EPI: A Carotid Stenting Trial for High-Risk Surgical Patients (BEACH) enrolled 747 high-risk patients from 47 US sites. CAS was performed with the Wallstent® and FilterWire EX/EZ systems (Boston Scientific, Nattick, MA) (9). The study included three groups: 189 roll-in patients, 480 pivotal group patients, and 78 registry patients with bilateral carotid stenoses treated by staged sequential CAS. The overall 30-day composite MAE was 5.8% (death 1.5%, stroke 4.4%, MI 1.0%), with no significant difference between the three groups. There were 25.3% symptomatic patients, and they had higher 30-day stroke rate than asymptomatic patients (7.4% vs. 3.4%, p = 0.038), but there was no difference in the 30-day composite MAE rates (7.9% vs. 5.0%). The 1-year endpoint of 30-day MAE plus ipsilateral stroke or neurologic death from 31 days to 1 year was 8.9% for the pivotal group, and repeat revascularization rate was 4.7% (10).
CABERNET (Unpublished Data)
The CABERNET (Carotid Artery Revascularization using the Boston Scientific EPI FilterWire EX/EZ and the Endo-Tex NexStent) trial enrolled 454 high-risk patients (24% were symptomatic) from 19 sites. The 30-day composite of death, stroke, and MI was 3.9% (death 0.5%, major stroke 1.3%, minor stroke 2.1%, MI 0.2%). The first primary endpoint of 30-day death, stroke, and MI, plus ipsilateral stroke from 31 days to 1 year was 4.5%. The second primary endpoint of all death, stroke, and MI at 1 year was 11.5%. At 3 years, the major stroke rate was 2.8%, and the ipsilateral stroke rate was 4.9%. These results were presented in the Transcatheter Cardiovascular Therapeutics (TCT) meetings in 2005 and 2007. The FDA subsequently approved the NexStent and FilterWire platform in December 2006. However, it was subsequently taken off the market, and is currently not available.
CREATE
The Carotid Revascularization With ev3 Arterial Technology Evolution (CREATE) trial enrolled 419 high-risk patients in 32 centers between April and October 2004. CAS was performed with the Protégé carotid stent and the Spider FX OTW filter EPD (ev3 Inc., Plymouth, MN) (11). Technical success was achieved in 97.4% of patients. Symptomatic patients represented 7.4% of the cohort and 24% of procedures were performed for patients with restenosis after prior CEA. The primary composite endpoint of death, stroke, and MI at 30 days occurred in 6.2% (death 1.9%, nonfatal stroke 3.3%, and MI 1%). In their multivariate analysis, the duration of filter deployment, symptomatic status, and baseline renal insufficiency were independent predictors of stroke. Patients who had their filter EPD deployed for over 20 minutes had almost double the risk of stroke and death compared to patients with filter deployment times >20 minutes (12). This was the first study to show that prolonged filter deployment time predicted higher complications with CAS, although it is not certain whether the extended filter time was causally related to complications, or simply a marker of the risk of complications. CREATE 2 studied the use of the AccuLink™ stent and the rapid-exchange version of the SPIDER filter. Of the 160 highrisk patients enrolled, the 30-day composite death, stroke, and MI event-rate was 5.6% (13). The SpiderRX device was FDA approved for CAS in February 2006 and the Protégé stent in January 2007. Following FDA approval, patients are currently being enrolled in the CREATE Post Approval study.
EMPiRE (Unpublished Data)
The EMPiRE study was a prospective, single-arm, multicenter registry that evaluated the safety and efficacy of the GORE Flow Reversal System during CAS in patients who were deemed high-risk for CEA. The study included 245 patients enrolled at 28 sites from July 2006 to July 2008, and preliminary results were reported at the TCT meeting in October 2008. The mean age of the patients was 70, of which 16% were octogenarians and 32% were symptomatic. The mean procedural time was 80 minutes (ranging from 25 to 345 minutes), with a mean flow reversal time of 15 minutes. Technical success rate with the GORE Flow Reversal System was 96.3%, with 2.4% of patients not being able to tolerate the procedure. The mortality rate was 0.8%, the death and stroke rate was 2.0%, and the MAE rate was 3.7%.
EPIC (Unpublished Data)
The EPIC study (Evaluating the Use of the FiberNet Embolic Protection System in Carotid Artery Stenting) was also recently reported at the TCT meeting in October 2008. This study enrolled 237 highrisk patients (from 26 US sites) with carotid artery stenosis who underwent carotid stenting using the FiberNet Embolic Protection System (Lumen Biomedical, Plymouth, MN) and a variety of approved carotid stents. This system provides for distal protection as well as aspiration of embolic debris. The technical success rate was 97.5%, with a FiberNet device success rate of 94.1%. The mean age of the patients was 74, 21.1% were octogenarians and 20% were symptomatic. The 1-month complication rates were low: mortality 0.4%, stroke 2.1%, MI 0.9%, with overall MAE of 3.0%.
MAVErIC (Unpublished Data)
The MAVErIC (Medtronic Self-Expanding Carotid Stent System with Distal Protection in the Treatment of Carotid Artery Stenosis) trial enrolled 99 high-risk CAS patients from 16 centers in the feasibility phase (MAVErIC 1) and 399 patients from 40 centers in the pivotal trial (MAVErIC 2) using the Exponent® self-expanding stent and GuardWire® distal balloon occlusion protection device (Medtronic, Minneapolis, MN). Within the pivotal group, 22% of patients were symptomatic and 34% had restenotic lesions after prior CEA. The 30-day death, stroke, and MI event-rate was 5.3% (death 1%, stroke 3.3%, and MI 2.0%) in the pivotal MAVErIC 2 trial presented at TCT 2004. The 1-year death, stroke, and MI event-rate from the feasibility MAVErIC 1 trial was 5.1%. The 1-year results for MAVErIC 2 have not been reported.
There is also the MAVErIC 3 study, which is intended to recruit 413 CAS patients in the United States using the Exponent® stent and a different EPD, the Interceptor Plus Carotid Filter system (also by Medtronic). In the smaller MAVErIC International Study that evaluated this CAS platform in 51 patients from 11 centers in Europe, Canada, and the Middle East (14), the 30-day MAE rate was 5.9% (death 2%, stroke 3.9%, MI 2%) and 1-year MAE rate was 11.8% (death 3.9%, stroke 5.9%, and MI 5.9%).
MO.MA (Outside the United States [OUS] data)
The MO.MA registry evaluated the use of the proximal occlusion Mo.Ma (Invatec) device among 157 patients who underwent CAS using a variety of approved carotid stents in 14 European centers from 2002 to 2003. This study did not exclusively enroll high-risk patients (75.2% were high-risk and 19.7% were symptomatic). Patients with contralateral carotid occlusion, severe external carotid artery disease, or proximal common carotid artery disease were excluded from this study. The Mo.Ma device was successfully used in 96.8% of patients, and a filter EPD was used in the unsuccessful cases. The mean duration of flow blockage was 7.6 minutes. The 30-day death and stroke rate was 5.7% (death 0.6%, major stroke 0.6%, minor stroke 4.5%), with no MI (15). A US IDE high surgical risk registry is completing enrollment.
PASCAL (OUS, Unpublished Data)
The PASCAL (Performance and Safety of the Medtronic AVE Self-Expandable Stent in Treatment of Carotid Artery Lesions) study was a European multicenter registry of 115 highrisk patients undergoing CAS with the Medtronic Exponent® stent and any CE Mark–approved EPD. The reported 30-day death, stroke, and MI rate was 8% (13). Although the study commenced in 2001, the results remain unpublished.
PRIAMUS (OUS)
The PRIAMUS (Proximal Flow Blockage Cerebral Protection during Carotid Stenting) study evaluated the Mo.Ma (Invatec) device in 416 CAS high-risk patients from four Italian centers from 2001 to 2006 (16). The study included 63.5% symptomatic patients, and did not exclude patients with contralateral carotid artery occlusion. The 30-day death, stroke, and MI event-rate was 4.6% (death 0.5%, major stroke 0.2%, minor stroke 3.8%, and no MI).
SECURITY (Unpublished Data)
The SECURITY (Registry Study to Evaluate the EmboShield™ Bare Wire Cerebral Protection System and Xact® Stent in Patients at High Risk for Carotid Endarterectomy) trial enrolled 305 high-risk patients from 29 US centers and 1 Australian center using the Xact® stent and EmboShield™ EPD (Abbott Vascular). Technical success was observed in 96.7%, and 21% of patients were symptomatic. The 30-day composite rate of death, stroke, and MI was 7.5% (death 1.0%, major stroke 2.6%, minor stroke 4.3%, and MI 0.7%). In comparison to historical weighted controls for CEA, the primary endpoint of 30-day MAE plus ipsilateral stroke from 31 days to 1 year was lower with CAS (8.5% vs. 14%) (17). The results were presented at TCT in 2003 and subsequently submitted to the FDA and granted approval in September 2005.
RANDOMIZED CAS VERSUS CEA STUDIES
The earliest randomized trials comparing CAS to CEA were fraught with limitations and high complication rates for CAS. These early trials utilized outmoded carotid angioplasty approaches, lacked consistent use of both stents and EPDs, and included inexperienced interventional operators. In contradistinction, surgical operators were carefully chosen and most had to have demonstrated adequate CEA procedural volume and low complication rates. As such, most of these published comparative studies were of poor Scientific quality and do add to the comparison of contemporary CAS versus CEA. Most would agree that comparing procedural outcomes of interventionists and surgeons of divergent skills/experience is not a reasonable comparison of the outcomes. CAS outcomes did not, until fairly recently, demonstrate a reduction and leveling off of adverse outcomes, likely due to better operator experience and patient selection reflecting an ongoing refinement of the field. Many of the earlier randomized outcomes, then, represent a technique, and technology, in evolution and distinctly different in that regard to a more than 50-year-old operation like endarterectomy that showed a similar early experiential development. We will briefly review the early trials followed by more recently published randomized CAS versus CEA trials.
The “Stopped” Trial (1998, OUS)
This was a single-center prospective, randomized trial from Leicester, UK that started in 1996 and was originally intended to randomize 300 symptomatic patients with carotid stenosis >70% (3). However, the trial was stopped prematurely due to an excess of complications in the angioplasty arm after 23 patients were enrolled, of which only 17 patients received their allocated revascularization. There were 5 stroke events among the 7 patients who underwent carotid angioplasty, compared with no stroke events among the 10 CEA procedures. The poor outcome in the angioplasty arm is considered a marked outlier and was attributed to inexperienced operators and suboptimal technique and equipment (no EPD use), in an era when CAS was still in its infancy. Due to the extensive limitations in this small single-center study, no appropriate conclusions could be drawn.
CAVATAS (1994 to 1997, OUS)
The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) randomized standard surgical risk patients to carotid angioplasty or CEA at 22 centers throughout Europe, Canada, and Australia from 1992 to 1997 (18). This study excluded high-risk patients and enrolled predominantly symptomatic patients (90% had neurologic symptoms within 6 months). There were 246 patients who underwent CEA and 240 had angioplasty without embolic protection (only 26% had stents, which were placed after 1994). The primary endpoint of 30-day MAE rate of death plus any stroke was 9.9% with CEA and 10% with angioplasty, and the death and disabling stroke rate was similar (6%). At 3 years, the rate of death or disabling stroke was also similar, 14.3% for angioplasty and 14.2% for CEA. This study has been criticized for a number of important limitations and practice that does not reflect current standards: formal sample size calculation was not performed, the 30-day MAE rates were unacceptably high in both arms (especially since high-risk patients were excluded), stents were used in only 26% of angioplasty cases, and no EPDs were used. In spite of the higher than expected event rate in both arms, this was the first randomized trial between surgical and nonsurgical management of extracranial bifurcation carotid disease to show that an endovascular approach might be reasonable.
The Wallstent Trial (1997 to 1999, US, Unpublished)
The Wallstent study randomized 219 symptomatic patients with standard surgical risk and carotid stenosis >60% to CEA versus CAS with the Wallstent® endoprosthesis (Boston Scientific) without EPD. The primary endpoint of ipsilateral stroke, procedure-related death, or vascular death within 1 year was 12.1% with CAS versus 3.6% for CEA (p = 0.022). The rate of any stroke plus death at 30 days was also higher with CAS (12.1% vs. 4.5% for CEA, p = 0.049). It was concluded that CAS was not equivalent to CEA in symptomatic low-normal risk patients, and the study was subsequently terminated prematurely before the planned enrolment of 700 patients. However, the study only has limited applicability given that inexperienced operators performed these CAS procedures, and dedicated carotid stent equipment and EPDs were not used. The study was only published in abstract form (19).
Community (Kentucky) Trial (1998, US)
This was a single-center randomized comparison of CAS (without EPD) versus CEA in a community hospital in Kentucky. In the first group consisting of 104 symptomatic patients with carotid stenosis >70% (“Community A”), 53 were randomized to CAS with the Carotid Wallstent® (Boston Scientific) and 51 to CEA (20). The event rates were low in both arms with one death in the CEA group and one transient ischemic attack (TIA) in the CAS group. There was a trend toward earlier hospital discharge with CAS (1.8 days vs. 2.7 days). In the second group (“Community B”) consisting of 85 patients with asymptomatic carotid stenosis >80% randomized to CAS or CEA (21), the event rates were extremely low with no procedure-related death or stroke in either arm. At 2 years, the vessel patency rates were similar between groups with no additional strokes. This study was small and precluded definitive conclusions, but provided a basis for future trial design.
CARESS (1999 to 2002, US)
CARESS (Carotid Revascularization Using Endarterectomy or Stenting Systems) was a multicenter, prospective, nonrandomized phase I clinical trial to compare CAS to CEA, and to provide an estimate of the 30-day death and stroke eventrate for future power calculations for a larger phase II clinical trial (22, 23). Treatment selection was based on patient and physician preference, with planned enrolment ratio of 2:1 for CEA to CAS. A total of 254 patients underwent CEA and 143 patients underwent CAS using the monorail Wallstent® (Boston Scientific) and GuardWire Plus EPD (Medtronic). Of the entire cohort, 85% were high-risk and 32% were symptomatic. The overall baseline characteristics were similar between the two groups except for a greater percentage of previous carotid revascularization in the CAS group. There was no significant difference in the combined death/stroke rate at 30 days (3.6% CEA vs. 2.1% CAS) or at 1 year (13.6% CEA vs. 10.0% CAS). There was also no significant difference in the composite of death, stroke, or MI at 30 days (4.4% CEA vs. 2.1% CAS) or 1 year (14.3% CEA vs. 10.9% CAS). The secondary end points of restenosis, repeat carotid revascularization, or change in quality of life were also not statistically different.
SAPPHIRE (2000 to 2002, US)
The SAPPHIRE (Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy) trial was the first contemporary randomized study to compare CAS with a dedicated stent system and EPD with CEA (24). This study included patients with symptomatic carotid stenosis >50% or asymptomatic stenosis >80%, who were high-risk patients for CEA as dictated by anatomic factors and medical comorbidities (Table 9.2). Patients were recruited from 29 US sites, and were reviewed by a multidisciplinary team (consisting of neurologist, vascular surgeon, and interventionalist) prior to enrolment. Patients who were deemed too high-risk for CEA were entered into a CAS registry, and patients deemed too inappropriate for CAS were entered into a CEA registry. Both surgeons and interventionalists participating in this trial had to have met the AHA guidelines for achieving periprocedural stroke and death rates of >6%. The Precise™ nitinol selfexpanding stent and AngioGuard™ filter EPD (Cordis Corporation, Johnson & Johnson) were used. The primary endpoint was a composite of death, stroke, and MI at 30 days, plus death or ipsilateral stroke between 30 days and 1 year. This study was designed to show that CAS was noninferior to CEA, and was terminated early in 2002 when enrolment slowed and when an interim analysis showed that the condition of noninferiority was met.
There were 307 patients randomized (156 underwent CAS and 151 underwent CEA), and 413 patients were entered into the stent (n = 406) and CEA (n = 7) registries. In the randomized study, 29.9% of patients were symptomatic in the CAS arm, and 27.7% in the CEA arm. Technical success with AngioGuard™ deployment was achieved in 95.6% of cases. The 30-day rates of death, stroke, and MI were 4.4% for CAS and 9.9% for CEA (p = 0.06). The 1-year primary endpoint occurred in 12.2% of patients with CAS versus 20.1% with CEA (p = 0.004 for noninferiority). In the noninferiority analysis, the p value was 0.053 for intention-to-treat analysis and 0.048 for pertreatment analysis. The 1-year target vessel revascularization rate was lower with CAS (0.6% vs. 4.3%, p = 0.04), as was the incidence of cranial nerve palsy (0% vs. 5.3%, p = 0.003) compared with CEA. In the high-risk CAS registry, the 30-day MAE rate was 7.8%.
These results have established CAS as a valid alternative to CEA in high-risk patients, and led to the FDA approval of CAS for this indication. Although this study is a widely accepted landmark CAS trial, there have been several criticisms of its limitations (25,26). The exclusion of a large proportion of patients from the randomized arm deemed too high-risk for CEA (entered into the CAS registry) was felt to have been biased against the types of patients enrolled into the randomized arm. However, the SAPPHIRE investigators argued that participating surgeons in this study were actually more experienced than the average, with a median volume of 30 cases annually, and the rate of cranial nerve palsy was lower than in North American Symptomatic Carotid Endarterectomy Trial (NASCET) (5.3% vs. 7.6%). The inclusion of periprocedural MI into the composite primary endpoint has also been criticized, as this endpoint was not included in traditional CEA trials. In a separate analysis excluding the 30-day MI endpoint, the SAPPHIRE event-rate was 5.5% with CAS and 8.4% with CEA (p = NS). Given that MI is an important morbidity frequently associated with carotid revascularization of this highrisk atherosclerotic population, its inclusion in the primary endpoint appears appropriate. Another criticism was the inclusion of a low percentage (~28%) of symptomatic patients in the study, and the lack of a medical treatment arm to help demonstrate the utility (or lack thereof) of revascularization in this high-risk population of mostly asymptomatic individuals.
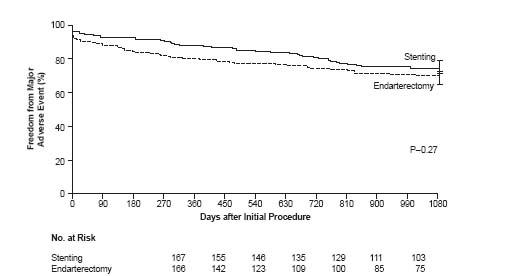
Criticisms aside, this was a well-conducted randomized controlled trial of contemporary CAS against CEA in high-risk patients, utilizing operators with established experience and procedural outcomes, and inclusion of routine neurologic evaluations postprocedure. Long-term 3-year outcomes have been recently published, revealing no difference in the prespecified secondary endpoint of the composite of death, stroke, or MI within 30 days, or death or ipsilateral stroke between 31 and 1080 days between the treatment groups (24.6% with CAS vs. 26.9% with CEA) and confirming long-term durability and stroke prevention efficacy of CAS equivalent to CEA (Fig. 9.1) (27). Thus, based in part on this landmark study, CAS was established as a viable alternative to CEA for highrisk patients, and arguably as the revascularization of choice in such individuals with anatomy favorable for an endovascular approach.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree