Chapter 19
Arteriography
Robert B. Mclafferty
Based on a chapter in the seventh edition by Nabeel R. Rana and Robert B. McLafferty
Despite many advances in the quality and availability of less invasive arterial imaging modalities, arteriography remains the “gold standard.” Alternative modalities, such as duplex arterial mapping, computed tomographic angiography (CTA), and magnetic resonance angiography (MRA), are being used with increasing frequency because of improved image quality and minimal risk.1–6 For some clinical scenarios, such as duplex surveillance of lower extremity vein bypass grafts, these imaging techniques have supplanted arteriography as the preferred test.7–9 However, with many first-line treatments being endovascular therapies, arteriography has returned to the forefront for further development of advanced imaging techniques and has become an indispensable hands-on tool for the vascular surgeon. A comprehensive understanding of basic to advanced techniques permits a wide array of arteriographic imaging studies to be performed optimally. In addition, a wide breadth of knowledge about arterial anatomy, equipment and materials, potential complications, and sources of interpretation error remains paramount for becoming an expert in performing arteriography.
The goal of this chapter is to impart a broad overview of modern arteriography. Clinical acumen about vascular disease and operative experience also play important roles in determining how to optimize arteriographic imaging. This chapter provides evidence-based guidance as well as practical experience for safe performance of high-quality arteriography.
Equipment
The task to determine what equipment is needed to perform high-quality arteriography can seem overwhelming. The necessary components can be divided into venue, imaging hardware, catheters, contrast agents, devices for delivery of contrast agents, and digital software used to assist in the processing of arteriographic images. Image processing closely relates to techniques of arteriography and is discussed later in the chapter.
Operating Room versus Imaging Suite
In past days, vascular surgeons felt most at ease in an operating room and, in many cases, a specialized cardiovascular operating room. That situation has changed dramatically, with the large majority of vascular surgeons now feeling equally comfortable performing endovascular procedures and coordinating a care team in other venues. Such procedures were once limited to the operating room with a portable fluoroscopy unit. However, many hospitals began accommodating vascular surgeons and allowing them to use venues with fixed-mount imaging units, such as the cardiac catheterization laboratory or the interventional radiology suite. Perhaps we have come full circle as hospitals are increasingly constructing combined operative-endovascular suites to specifically accommodate the modern needs of the vascular surgeon. A state-of-the-art fixed-mount imaging unit combined with all the features of an operating room, a fully equipped endovascular stock (catheters, guide wires, and devices), and a trained staff that is comfortable with endovascular and open vascular surgery should set the standard for which vascular surgeons strive in refining their place of practice.
The advantages of an operative-endovascular or hybrid suite are clear. Although combined endovascular and open procedures are not common in view of the total volume of angiographic procedures, there has been a tremendous increase in aortic endograft procedures alone, which requires adept staff and a sterile environment for operative exposure of the femoral arteries and superior imaging capabilities for graft positioning and deployment. Complications that arise during any endovascular procedure, albeit rarely, can be managed by immediate and seamless conversion to open surgery as opposed to the need for emergency transport from an angiography suite to the operating room. Currently, emphasis should also be placed on developing specialty operative-endovascular teams to further meet the procedural needs of the vascular surgeon.
Fixed-Mount versus Portable Equipment
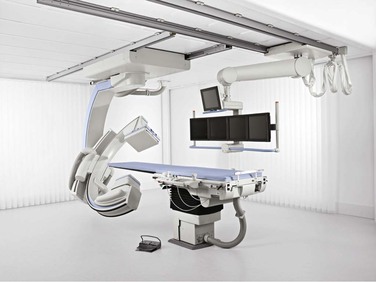
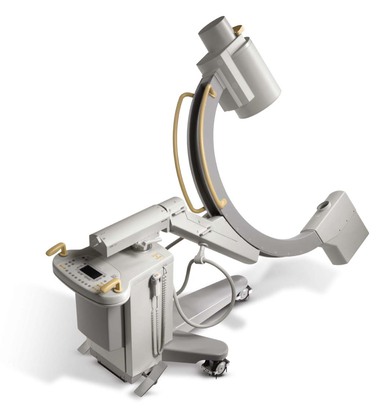
The two principal types of radiographic imaging units that can provide state-of-the-art arteriography are the fixed-mount unit physically attached to one suite (Fig. 19-1) and the portable fluoroscopy unit equipped with wheels so that it can be moved from room to room (Fig. 19-2). Each type has the basic components, including a power generator for fluoroscopy, a C-arm with image intensifier, screens for viewing real-time and reference images, and digital image processing software. In experienced hands, both types of imaging units have their advantages and disadvantages.
In general, fixed-mount units have more powerful generators that allow more detailed imaging. More power allows greater depth of fluoroscopic penetration and thus the ability to discern finer detail. Depending on the types of imaging units compared, fixed-mount units can provide upward of 10 times the resolution of portable units. Although this greater resolution may not be needed in persons of ideal body weight, increased power for improved penetration is important when arteriography is performed on obese patients. Another distinct advantage of a fixed-mount unit is a larger field of view. Modern fixed-mount units now have flat detector image intensifiers as large as 19 inches (48-cm image projection). This increased size translates into the ability to cover more imaging area during arteriography with less radiation exposure and less total volume of contrast material. In addition, fixed-mount units have tableside controls that allow precise and easily directed movement of the C-arm and table. This feature is useful during visceral and vertebral arteriography inasmuch as subtle manipulations in C-arm angulation can result in significantly improved arterial visualization.
A portable fluoroscopy unit designed for high-quality arteriography is advantageous by the mere ability to move the equipment from room to room. This may be important in trying to fulfill the needs of different types of vascular surgical practices, operating rooms, specialty procedure rooms, and intensive care units. Depending on a patient’s clinical condition, bringing the imaging equipment to the patient can be easier, safer, and more practical than bringing the patient to a suite with a fixed-mount unit. Typically, portable fluoroscopy units require only one technician in addition to the vascular surgeon for arteriography to be adequately performed. Fixed-mount units require, in addition to the angiographer, three or four specially trained staff, including a technician who is gowned and gloved with the vascular surgeon, a circulator attending to material needs, a technician coordinating image acquisition and processing, and a certified nurse who is responsible for monitoring the patient and administering medications. Finally, portable units are much less costly than fixed-mount units. Not only are fixed-mount units expensive (ranging from $1.5 to $3 million), but construction of such a suite may require major structural modifications to an existing room, which can be a significant additional cost.
As the necessity of arteriography continues with the technologic advancement of complicated endovascular procedures such as fenestrated endografting, so too will the need for the use of fixed-mount imaging units with advanced capabilities. These procedures will need enhanced angle abilities, three-dimensional capabilities, better resolution, and complementary relationships with other simultaneous imaging technologies to allow precise abilities to image and subsequently to position all forms of devices. Furthermore, this in turn will continue to minimize radiation exposure to both the patient and the hybrid operating room team.10–13
Catheters
The type of catheter used also plays an important role in optimizing the arteriographic image and avoiding injury to the vessel. Intra-arterial catheters come in virtually all shapes, sizes, and lengths. Given this variety, attention should be paid to whether the catheter has just an end hole or multiple side holes in addition to the end hole (Fig. 19-3). The latter, known as a flush catheter, is designed for safe, quick, and even dispersion of contrast material at high injection pressures to image large arteries with high flow. End-hole catheters, with their greater variety of shapes, are often all that can be used after remote arterial access is achieved. Care should be taken whenever a power injection is performed with an end-hole catheter in a medium-sized muscular artery. Problems may include loss of a tenuous catheter crossing because of pressure forcing the catheter out of the arterial lumen, creation of intimal flaps, and arterial rupture. Direct power injection into very small arteries, such as the vertebral or tibial arteries, should be avoided with any type of catheter. Hand injection with a manifold should also be performed gently to avoid injury to these vessels.
Contrast Agents
The concept of angiographic imaging relies on the presence of a contrast agent within the vessel to be visualized at the time of radiographic exposure. To maximize vascular anatomic detail, this agent needs to have a radiodensity that is distinct from that of the tissue being imaged. Typical contrast agents have greater radiodensity than surrounding tissue and therefore appear markedly darker on imaging. However, an agent significantly less radiodense than surrounding tissue, such as carbon dioxide (CO2) gas, can also provide adequate contrast to image intravascular anatomic detail,14,15 albeit with less quality in most cases. The underlying concept is to provide adequate visual contrast between the intravascular space and the extravascular space. Selecting the appropriate contrast agent and adjusting the imaging equipment and technique accordingly are essential for patient safety and imaging quality.
Iodinated Contrast Agents
Conventional contrast agents are composed of molecular compounds containing iodine. These iodinated agents can be categorized as ionic or nonionic.
Ionic contrast agents dissociate into anions and cations when they are placed in solution, which includes flowing blood. The anion is a benzene ring fully substituted with three iodine atoms. Iodine atoms absorb the x-ray photons and are responsible for contrast visualization, or radiopacity, of the artery visualized. The cation can be sodium, methylglucamine, or a combination thereof. When iodinated contrast agents dissolve, their osmolality doubles because of the presence of two ions. The osmolality of these agents ranges from 1500 to 1700 mOsm, thus making them significantly more hyperosmolar than plasma (285 mOsm).
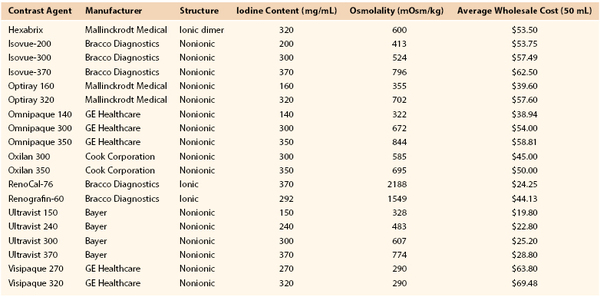
Nonionic contrast agents have considerably less osmolality. Because dissolution of the benzene compound into two ions is prevented, the osmolality remains half that of ionic contrast agents. However, because the number of iodine atoms remains the same, radiopacity remains comparable to that of conventional ionic agents but with much less ionic charge. Osmolality can also be reduced by a compounding method that creates a dimeric ionic contrast agent, whereby two benzene rings are attached to each cation. With this formulation, twice the number of iodine atoms are present with the same level of osmolality (ranging from 320 to 880 mOsm). Doubling of the iodine atoms leads to a greater amount of x-ray photon absorption, and thus comparable arteriographic images are achieved with less volume of contrast material. Table 19-1 outlines important information about commonly used ionic and nonionic contrast agents. Comprehensive knowledge of contrast agents is vital to performing arteriography safely.
Carbon Dioxide Arteriography
Another contrast agent with novel properties and applications is CO2.14,15 Injection of this gas with its decreased radiodensity creates radiographic contrast by transiently displacing blood from the artery being imaged. Historically, CO2 arteriography has had limited use because of poor imaging quality in comparison to conventional contrast agents. However, with improved equipment, technology, and image processing software, coupled with the significant number of vascular patients who have compromised renal function, CO2 arteriography has expanded to a wide variety of cases, and it can be used with much less reservation and hesitation than in the past.15–17 Digital subtraction technology has greatly enhanced anatomic definition and visualization with this contrast agent. Despite improved techniques, however, image quality still remains inferior to that of conventional contrast agents.
Many techniques can enhance the quality of CO2 arteriography. Because of the image settings developed to enhance visualization of intravascular CO2, the presence of bowel gas can especially degrade image quality in this acquisition mode. Therefore, the patient should begin a clear liquid diet the day before the procedure and take 80 mg of simethicone 30 minutes before the procedure. During the procedure, 0.5 mg of glucagon may be given intravenously if significant bowel gas persists. Because CO2 rapidly displaces blood and then dissolves quickly, frame rates are increased during image acquisition (four to eight frames per second). Modern arteriographic imaging systems now feature prescribed settings that depend on the type of contrast agent and area of the body to be imaged. Further image enhancement can be achieved by selective catheterization and magnified views of smaller caliber vessels (Fig. 19-4). Contrast injections may be performed rapidly by hand or with an automatic pump system. They should be spaced 3 to 5 minutes apart to allow complete dissolution before the next injection. Overestimation of the degree of stenosis can occur when one is unable to completely fill the diameter of the artery with CO2.18 For lower extremity arteriography, Trendelenburg positioning or elevation of the extremity to about 20 to 30 degrees will slow distal blood flow and promote concentration of the CO2, thus slowing the dispersion rate as CO2 exits the catheter and travels distally.
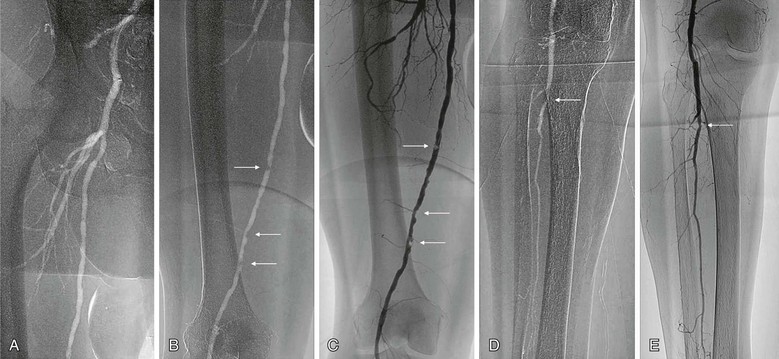
Figure 19-4 A, Angiogram of the femoral bifurcation visualized with CO2 contrast and selective placement of the catheter in the common femoral artery. B, Opacification of the superficial femoral artery reveals multiple stenoses (arrows). C, Comparison of the same vessel opacified with iodinated contrast material shows much better characterization of each stenosis (arrows). D, Distal views show relatively well visualized popliteal and single-vessel peroneal runoff with CO2; significant disease is evident at the proximal peroneal artery (arrow). E, Again, better characterization with iodinated contrast material reveals only a mild stenosis at the proximal peroneal artery (arrow).
The CO2 delivery system includes a 30- to 60-mL Luer-Lok syringe, tubing with a two-way distal stopcock and two one-way check valves, a 1500-mL fixed-volume gas bag, and the CO2 tank (Fig. 19-5). After assembly of the system, purging of the tubing with three syringe volumes (90 to 180 mL) of gas prevents the possibility of accidental injection of air. Nitrogen gas in ambient air is not soluble and can lead to gas embolization. Once the gas bag is filled with CO2 directly from the gas tank, the syringe is pulled back to be filled with gas from the gas bag. Because of a one-way valve distal to the syringe, only gas from the bag can be withdrawn. Another reversed one-way valve proximal to the syringe forces gas to travel only to the patient on injection.
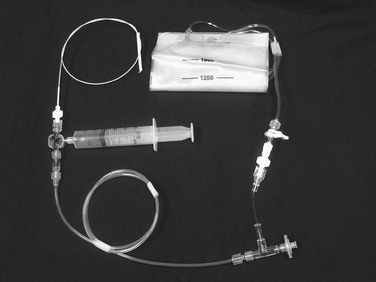
Figure 19-5 CO2 system connected to a diagnostic catheter. The delivery system uses a three-way stopcock, check valve, and filter port connected to the gas tank (not shown). A 1500-mL bag is attached to the delivery system with low-pressure tubing and a two-way stopcock. A large Luer-Lok syringe is required for hand injection.
Gadolinium Arteriography
Gadolinium (basic element atomic number 64) is classified as a rare earth metal. Gadolinium salts, such as gadolinium trichloride, are water-soluble compounds that maintain paramagnetic properties at the body’s neutral pH. This property makes gadolinium-based contrast agents ideal for use in magnetic resonance imaging. The U.S. Food and Drug Administration has approved five gadolinium-based contrast agents for intravenous use with magnetic resonance imaging (Omniscan, OptiMARK, Magnevist, ProHance, and MultiHance). Intra-arterial injection of these agents represents off-label use, although gadolinium-based contrast agents have been substituted for iodinated contrast agents during the performance of arteriography. The risks and limitations of gadolinium-based contrast agents can be found in Chapter 23.
Devices for Injection of Contrast Agents
Injection of contrast material into a catheter can be performed with a power injector or manually. For optimal arteriography, the ability to perform both methods is preferable because both have distinct advantages, depending on what arteries are being imaged.
Power Injection
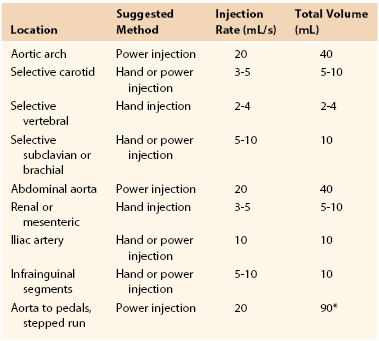
Power injectors allow rapid high-pressure injection of contrast material into the catheter. Precise control exists with regard to the pressure setting (psi), amount of contrast material used during a set period, timing of injection in relation to fluoroscopic imaging and catheter position, and rate of rise of the injection. Typically, use of a power injector allows rapid filling of large arteries at high flow rates. This produces equal distribution of contrast material and hence a higher quality image. Power injectors can also be used for prolonged injections, generally ranging from 3 to 6 seconds, when the catheter can be placed only in a very proximal position and the target artery is remote. Imaging of the dorsal pedal artery with the catheter placed in the ipsilateral common femoral artery is one such example. When a power injector is used, settings must be adjusted to manipulate pressure and the rate of rise of the injection, depending on the vessels being imaged. Large high-flow arteries require high pressure (800 psi) and rapid rise of the injectate to quickly disperse the contrast agent for a proper image. Smaller arteries, such as the superficial femoral artery, can be traumatized by such settings and require a lower pressure setting (400 to 600 psi) and reduced rate of rise of the injectate. For every power injection, the vascular surgeon will determine what volume of contrast material is to be given during what time. For example, typical aortic arch arteriography may require 20 mL of contrast material per second for a total of 2 seconds. Common jargon in the endovascular suite before such an injection would be “20 for 40,” or 20 mL of contrast material injected per second for a total volume of 40 mL.
Manual Injection
Manual injection of contrast material can be performed by use of a simple syringe with full-strength contrast agent or a manifold device that allows more precise and rapid loading of contrast material (Fig. 19-6). Manifolds are also equipped with the ability to dilute contrast material with saline, to evacuate air and liquid waste, and to perform real-time intra-arterial pressure measurements. The ability to perform high-quality arteriography with the use of dilute contrast material should not be underestimated. Contrast agents, many of which are nephrotoxic, should always be used conservatively regardless of renal function. Establishing this habit helps prevent injury to patients with mild renal disease not yet manifested by abnormal laboratory values. In smaller arteries (e.g., the common carotid artery or tibial arteries), 3 to 4 mL of diluted contrast material (1 : 1 mixture of contrast material and saline) should be sufficient to give a high-quality arteriographic image. The learning curve for maximizing proper use of a manifold is not very steep, and once it is mastered, optimal arteriographic images can be obtained rapidly with minimal volume of contrast material. Typical injection methods, rates, and volumes of contrast agent are detailed in Table 19-2.
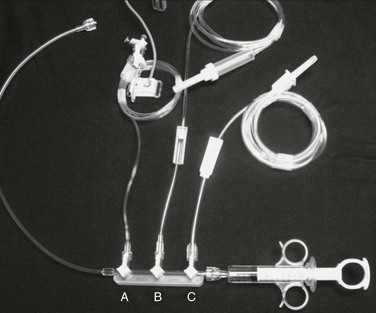
Figure 19-6 Rapid, convenient device for mixing and delivery of contrast material. The control syringe and manifold allow quick reloading after each injection of contrast agent, with one hand operating the syringe and the other regulating the proportions of contrast material (C) and saline (B) being drawn up via the stopcocks on the manifold. Between injections, the saline line can be left on flush to keep the catheter irrigated. The third stopcock (A) allows continuous monitoring of pressure when it is connected to a transducer.
Techniques
Arteriographic imaging in the modern era is synonymous with digital subtraction angiography (DSA). The basic concept behind DSA involves a recording technique by which the captured fluoroscopic image is amplified and digitized. With advanced computer data processing capability, multiple manipulations of the stored data can be performed to optimize image quality.
Image Processing
Some of the commonly used postprocessing capabilities are subtraction, masking, pixel shifting, and view tracing. Other tools, such as roadmapping and unsubtracted image referencing, can assist in guiding wires and catheters during the actual procedure.
Subtraction Tool and Masking
The subtraction tool is the single most effective characteristic of this technology that enhances and improves image quality. This technique subtracts all visible radiodensities on the current image to create a mask image, represented as a blank screen. Once the mask has been established, any motion in the field during the fluoroscopic run will be highlighted as a dynamic image on this blank background. All radiodense tissues subtracted on the mask image will remain subtracted as long as they remain in the same position and alignment as in the mask image. After subtraction is achieved and the mask image is created, the contrast agent is injected and visualized because it is radiodense and moves with blood flow through the vessels of concern. By subtraction of the surrounding tissue and vessel wall from the contrast images, the quality of visualization of intraluminal contrast is greatly enhanced. In an ideal image capture, there is no movement between creation of the mask and injection of contrast material. This would produce the purest image derived solely from flow of the contrast agent. However, such is often not the case, and unintended motion can greatly compromise imaging. If the mask has been created and the patient then changes position, breathes, or even has bowel peristalsis, any of this skeletal or visceral motion will be seen on the contrast images because it was not part of the original mask image. Therefore, it is essential for the patient to remain very still during image capture.
The concept of masking, as just discussed, can be used beyond the original subtraction maneuver to improve image quality. Any single image in the series during a contrast run can be designated the mask image. If the patient moves after creation of the mask, the mask can be reassigned to an image after the movement occurred but before or after contrast material is seen in the field of view, and this would create a new blank screen. The artifact from patient movement seen on the original contrast images could potentially be lessened or even eliminated by replacing the mask in this way. If an image with contrast already exposed is designated the mask image, this segment of contrast would be subtracted and not visualized on the final images. This is why the mask image should be the image before the contrast agent enters the field of view or after it has completely washed out. With this concept in mind, if motion is noted during the imaging run, the filming process should be continued until all contrast material is washed out so that these late images may be used as potential mask images. A trial of different mask images is often an effective way of finding the best possible result.
Pixel Shifting
Pixel shifting provides a valuable tool when artifactual movement occurs after contrast material has already entered the field of view. Instead of creating a new mask, one can take the existing mask and slide it in a bidirectional plane (vertical, horizontal, or both) to realign the surrounding tissue (Fig. 19-7). This realignment of the existing mask to the patient’s current position on the contrast image erases the discrepancy and minimizes the motion artifact. Some software programs allow automatic or manual pixel shifting. In the automatic mode, the computer will try to realign the mask image to create the best subtracted result for that particular region of the field. In manual mode, the pixel shifting can be done freehand in real time by sliding the cursor vertically, horizontally, or both until the optimal image is achieved.
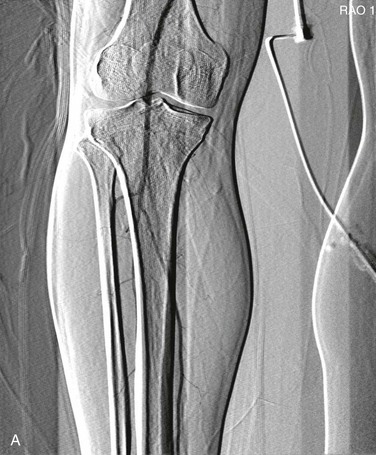
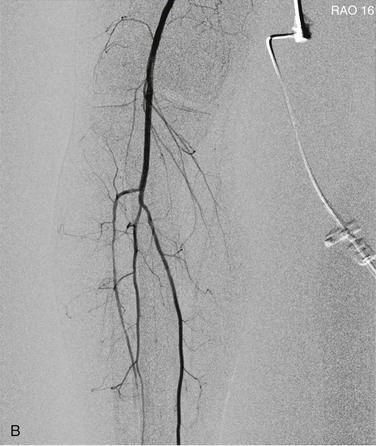
Figure 19-7 A, Patient motion during contrast opacification significantly degrading image quality. B, Pixel shifting during postprocessing realigns the mask image with the contrast image and thereby results in optimal visualization of the artery. If the motion occurs before contrast injection as opposed to during it, reassigning the mask image can accomplish a similar result. RAO, Right anterior oblique.
Because arteriography is a dynamic imaging modality, obtaining one static image from a series during injection of contrast material will often fail to provide complete visualization of the target vessel. As the contrast agent opacifies one segment of the blood vessel, a previous segment may already have washed out and a distal segment may not yet have filled with the contrast agent.
View Tracing
View tracing, sometimes referred to as opacification, allows consolidation or “stacking” of different time points by overlaying consecutive static images in a series to provide one cumulative arteriographic image. The first image in which contrast appears may be designated the first image in a view-traced series. Each subsequent image that is stacked over the previous one will show further progression of contrast and further visualization of the vessel distally (Fig. 19-8). However, with each additional view-traced image, any motion artifact will also be superimposed, thereby degrading the image more and more with sequential stacking. To circumvent this problem, each individual static image can first be optimized by pixel shifting to minimize artifact in the final cumulative image.
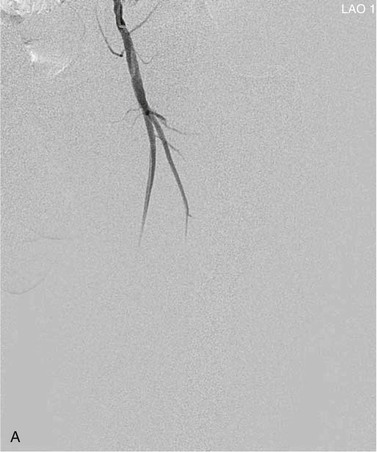
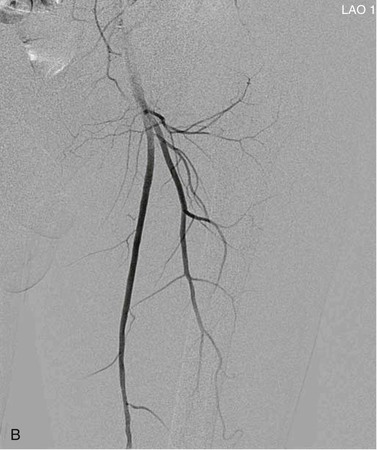
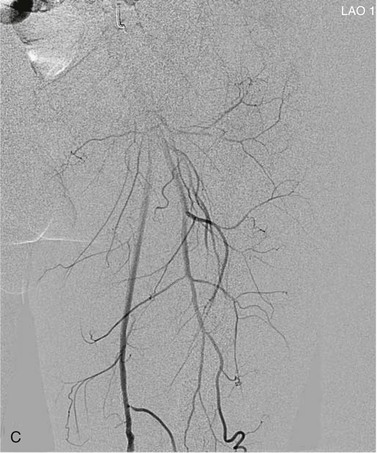
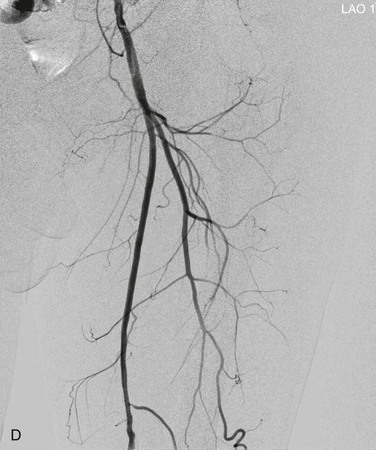
Figure 19-8 A, Femoral arteriogram early after injection of contrast material illustrating only proximal opacification of the vessels. B and C, Progress of contrast opacification through the femoral vessels with improved distal but poor proximal visualization. D, View tracing allows stacking of sequential images to give a comprehensive view of the vessels with all areas opacified during the different time points of the contrast run. LAO, Left anterior oblique.
After broad manipulations with the techniques just discussed, images can be further enhanced by finer adjustments. Image contrast, brightness, edge enhancement, sharpness, and even color of the contrast agent (image invert) can be modified to create a final optimized image. These finalized images can then be stored in the patient database along with the dynamic DSA runs or to any storage medium of choice.
Roadmapping and Measuring
Other useful techniques involving DSA can help with guidance during the actual procedure. Roadmapping allows a previously constructed image of a contrast-filled vessel to be displayed on the working monitor. Real-time fluoroscopic imaging can then be superimposed on the monitor so that the contrast image can be used as a “roadmap” to direct guide wires and catheters. This technique is effective as long as table and patient positions do not change. The roadmap mask is extremely sensitive to motion, and image quality will degrade during use. Another valuable guiding tool during arteriography is the simple use of unsubtracted image referencing. Disabling subtraction from a contrast image allows identification of native tissue reference points, most often bone landmarks, near vessel bifurcations, for example (Fig. 19-9). This image can then be displayed on a reference monitor alongside the working monitor so that the angiographer can manipulate catheters or wires on the basis of these reference points.
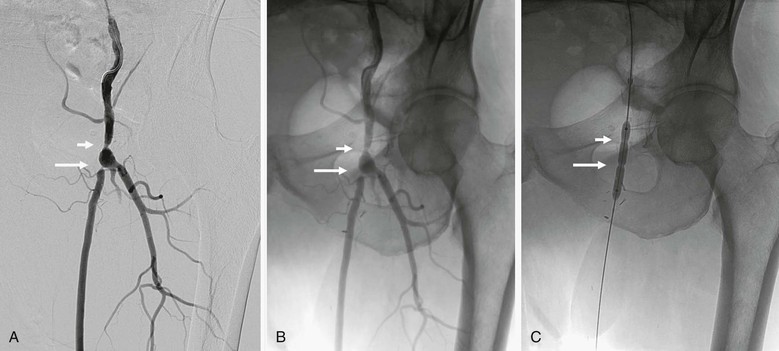
Figure 19-9 A, A femoral arteriogram to evaluate a lower extremity bypass graft shows a stenosis at the proximal anastomosis (short arrow) and a second stenosis in the proximal graft (long arrow). B, Viewing of the same image in an unsubtracted mode allows the use of bone structures, such as the pubic rami and obturator foramen, to serve as reference landmarks. C, On the unsubtracted reference image, localization and treatment of the target lesions can be performed accurately with guidance from these bone landmarks.
Finally, measurement tools are also frequently part of the software capabilities now available with DSA equipment. These tools can be autocalibrated on the basis of estimated distances from the image intensifier to the center of the patient’s body, or they can be manually calibrated by measuring an object with known dimensions, such as a quarter, and placing it on the patient’s body in the same field where measurements are desired. This allows more accurate interpretation of stenoses and vessel dimensions than is possible with mere visual estimation or “eyeballing.”19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree