We sought to determine the arterial mechanics at rest and during exercise in adolescents and young adults with complete transposition of the great arteries after arterial switch operation and their relations with neoaortic complications. Thirty patients (22 men) aged 16.2 ± 2.1 years and 22 controls (15 men) were studied. Central and peripheral arterial pulse wave velocities, carotid and radial augmentation indices, and central systolic blood pressure (cSBP) were determined by oscillometry and applanation tonometry, whereas arterial dimensions were measured by 2-dimensional echocardiography. Arterial strain, distensibility, and stiffness were determined at rest and during supine bicycle exercise testing. At rest, patients had significantly higher heart-carotid pulse wave velocity, carotid and radial augmentation indices, and cSBP than controls. At rest and during submaximal exercise, patients had significantly lower aortic strain and distensibility, greater aortic and carotid stiffness, and higher SBP than controls. Dilated aortic sinus found in 23 (76.7%) patients was associated with lower aortic distensibility, greater aortic stiffness, and higher cSBP at rest and lower aortic distensibility and strain at submaximal exercise. Significant aortic regurgitation found in 20% (6 of 30) of patients was associated with significantly higher neoaortic z scores. Multivariate analysis identified aortic stiffness at rest (β = 0.46, p = 0.003) and age at operation (β = 0.44, p = 0.004) as significant determinants of aortic sinus z scores. In conclusion, altered mechanics of the central arteries are present at rest and during exercise in adolescents and young adults after arterial switch operation. These findings may have important implications on progression of neoaortic root dilation, exercise recommendations, and medical therapy.
Since the first successful report almost 4 decades ago, arterial switch operation (ASO) has become the surgery of choice for complete transposition of the great arteries (TGA). Intermediate and long-term studies have shown good outcomes including a low late mortality, achievement of satisfactory exercise capacity, and preservation of systemic ventricular function. With the growing population of adolescents and adults after ASO, progressive dilation of neoaortic root and incompetence of neoaortic valve are becoming issues of concern. In this study, we sought to determine the arterial mechanics at rest and during exercise in adolescents and young adults with complete TGA after ASO and their relation with neoaortic root dilation and neoaortic valve regurgitation.
Methods
Thirty patients with TGA and normal left ventricular function who have undergone ASO for >10 years were recruited from the pediatric cardiac clinic. The following clinical data were collected: age at operation, follow-up duration, associated cardiac lesions, residual postoperative cardiac lesions, and the need for cardiac interventions after ASO. Twenty-two healthy subjects were recruited as controls. The body weight and height of all subjects were measured, and body surface area (BSA) was calculated accordingly. All the subjects underwent assessment of vascular function at rest and during supine bicycle exercise testing as described later. The Institutional Review Board approved the study and patients and parents of minors gave informed written consent.
Transthoracic echocardiography was performed using Vivid 7 ultrasound system (General Electric, Vingmed, Horten, Norway). Echocardiographic cine loops were stored on digital versatile discs for offline analysis using Echopac software (General Electric) by a single investigator (RHC). The average values of echocardiographic parameters from 3 cardiac cycles were obtained for statistical analysis.
Neoaortic dimensions at 4 levels, namely the annulus, sinus of Valsalva, sinotubular junction, and proximal ascending aorta, were measured from the parasternal long-axis view, normalized by BSA, and expressed as z scores. Severity of neoaortic valve regurgitation was assessed quantitatively by Doppler color flow mapping.
Elastic properties of the neoaorta were assessed both at rest and at submaximal exercise testing. Proximal ascending aortic systolic (AoS) and diastolic (AoD) dimensions were measured using M-mode echocardiography. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) of the right upper arm was obtained using an automated oscillometric device (Dinamap, Critikon, Inc., Tampa, FL). The following neoaortic mechanical parameters were derived: (1) strain = (AoS−AoD)/AoD × 100%, (2) distensibility = [2 (AoS−AoD)]/[AoD (SBP−DBP)], and (3) stiffness index = ln(SBP/DBP)/[(AoS−AoD)/AoD]. Stiffness index of the right carotid artery, assessed at about 1 cm proximal to its bifurcation, at rest and during exercise was similarly determined.
Regional stiffness of the central and peripheral arterial segments was assessed by measuring the heart-carotid, heart-femoral, brachial-ankle, and femoral-ankle pulse wave velocities (PWVs) using an automated device (VP-2000; Colin Medical Technology, Komaki, Japan). Briefly, the device captured simultaneously the electrocardiographic signals, phonocardiogram, bilateral brachial, and ankle blood pressure waves by oscillometry and carotid and femoral arterial pulse waves by applanation tonometry. For tonometric measurements, gentle compression of the arteries by the tonometer allows equalization of in-built pressure sensors with arterial circumferential pressure and derivation of an arterial pulse waveform. Pulse wave velocity was obtained by dividing the path length of pulse traveled by the transit time. Based on the carotid waveform, the carotid augmentation index (cAI) was calculated as the ratio of the amplitude of the pressure wave above its systolic shoulder to the total pulse pressure.
To estimate the central aortic pressure, the right radial arterial pulse waveform obtained by applanation tonometry (HEM 9000-AI; Omron-Healthcare, Kyoto, Japan) was analyzed. Based on the waveform, an early peak and a late systolic peak were identified for derivation of early radial systolic blood pressure (rSBP) and late radial SBP (rSBP2), respectively. The central systolic blood pressure (cSBP) was then derived from the rSBP2 as described, previously. A radial augmentation index (rAI) was calculated as [(rSBP2–DBP)/(rSBP–DBP)] × 100% and normalized to a heart rate of 75 beats/min.
Submaximal exercise testing was performed using a bicycle ergometer (Ergosana Schiller Semi-couch Safety Ergometer ERG 911 S/L, Swabian Alb, Germany). The subjects were asked to avoid caffeine-containing food or drinks and exercise on the day of study. Baseline assessment was performed after at least 5 minutes of rest. The initial workload was 25 W, with a stepwise increase of 25 W of workload at 2-minute intervals until a maximum of 150 W or when further increment of workload was not tolerated. The exercise test was terminated when 70% of age-predicted maximum heart rate was reached.
Data are expressed as mean ± SD unless otherwise stated. Demographic, echocardiographic, and vascular functional parameters of patients and controls were compared by unpaired Student’s t test and Fisher’s exact test where appropriate. Analysis of variance with 2 factors was used to determine the effect of submaximal exercise stress (exercise vs at rest) or the effect of grouping (patients vs controls) on the aortic and carotid vascular functional parameters. Pearson correlation analysis was used to assess relation between the neoaortic elastic indices and z scores of the neoaortic dimensions. Stepwise multiple linear regression analysis was used to identify significant determinants of neoaortic root dilatation. Statistical analyses were performed using SPSS version 19 (SPSS, Inc., Chicago, IL). A p value <0.05 was regarded as statistically significant.
Results
Of the 30 patients, 22 men and 8 women, 24 (80%) patients had an intact ventricular septum, whereas 6 (20%) had an associated ventricular septal defect (VSD). The median age at ASO was 14 days (range 7 to 220 days). Six patients underwent ASO beyond the neonatal period, all of whom had an associated VSD. Two of these patients had staged operation with initial pulmonary arterial banding before ASO. The patients were studied at 16.2 ± 2.1 (range 12.7 to 21.3) years of age. At the time of study, 6 patients had moderate to severe neoaortic regurgitation, 2 of whom were on enalapril therapy. Six patients had residual cardiac lesions, including 3 with mild supravalvar pulmonary stenosis, 1 with mild valvar pulmonary stenosis, 1 with right pulmonary arterial stenosis, and 1 with a small residual VSD. Intervention was required only in the patient with right pulmonary arterial stenosis who had undergone repeated balloon angioplasties.
The 22 controls, 15 men and 7 women (p = 0.76), were aged 15.6 ± 2.3 (range 12.1 to 20.3) years (p = 0.31). There were no significant differences in body weight (55 ± 2 kg vs 54 ± 1 kg, p = 0.89), height (165 ± 11 cm vs 164 ± 10 cm, p = 0.58), and surface area (1.59 ± 0.20 m 2 vs 1.58 ± 0.20 m 2 , p = 0.75) between the 2 groups.
Table 1 summarizes the PWVs at rest and radial pulse waveform parameters in patients and controls. At rest, patients had significantly higher heart-carotid PWV, cAI, cSBP, rAI, and adjusted rAI at 75 beats/min compared with controls. Other PWV parameters were otherwise similar between the 2 groups.
| Variable | Patients (n = 30) | Controls (n = 22) | p Value |
|---|---|---|---|
| Brachial-ankle pulse wave velocity (cm/s) | 1,055 ± 193 | 1,043 ± 145 | 0.80 |
| Femoral-ankle pulse wave velocity (cm/s) | 863 ± 146 | 828 ± 130 | 0.38 |
| Heart-femoral pulse wave velocity (cm/s) | 639 ± 69 | 625 ± 68 | 0.50 |
| Heart-carotid pulse wave velocity (cm/s) | 554 ± 87 | 476 ± 92 | 0.002 ∗ |
| cAI (%) | −25 ± 13 | −36 ± 15 | 0.006 ∗ |
| cSBP (mm Hg) | 110 ± 11 | 103 ± 9 | 0.020 ∗ |
| rAI (%) | 63 ± 13 | 55 ± 13 | 0.032 ∗ |
| rAI at 75 beats/min (%) | 62 ± 14 | 53 ± 13 | 0.037 ∗ |
Table 2 summarizes the arterial elasticity indices at rest and during exercise. Compared with controls, patients had significantly lower aortic strain and aortic distensibility and greater aortic and carotid stiffness index both at rest and during exercise. The SBP was also significantly higher in patients than controls at rest and during exercise.
| Variable | Rest | Submaximal Exercise | p Value | ||||
|---|---|---|---|---|---|---|---|
| Patients (n = 30) | Controls (n = 22) | Patients (n = 30) | Controls (n = 22) | Group Factor | Exercise Factor | Interaction | |
| Aortic strain (%) | 12.5 ± 4.3 | 21.6 ± 4.1 | 14.5 ± 4.8 † | 21.8 ± 4.0 † | <0.001 ∗ | 0.20 | 0.32 |
| Aortic distensibility (cm 2 /dynes × 10 −6 ) | 3.3 ± 1.1 | 6.9 ± 1.4 | 2.6 ± 0.9 † | 4.3 ± 1.4 † | <0.001 ∗ | <0.001 ∗ | 0.001 ∗ |
| Aortic stiffness index | 5.6 ± 2.1 | 2.7 ± 0.5 | 6.0 ± 2.3 † | 3.6 ± 0.7 † | <0.001 ∗ | 0.052 | 0.49 |
| Carotid stiffness index | 5.4 ± 1.2 | 4.3 ± 0.7 | 6.5 ± 2.4 | 4.4 ± 1.0 | <0.001 ∗ | 0.08 | 0.22 |
| SBP (mm Hg) | 121 ± 14 | 115 ± 12 | 169 ± 18 | 162 ± 18 | 0.024 ∗ | <0.001 ∗ | 0.77 |
| DBP (mm Hg) | 65 ± 5 | 66 ± 6 | 76 ± 10 | 74 ± 9 | 0.52 | <0.001 ∗ | 0.37 |
† Data available for 22 of 30 patients and 20 of 22 controls because of limited acoustic windows during exercise.
Aortic distensibility decreased significantly during exercise (p <0.001 for exercise factor). Significant interaction between group and exercise factors for aortic distensibility suggested differences in magnitude of changes with exercise between the 2 groups ( Figure 1 ). Whereas almost uniform reduction of aortic distensibility was found in control subjects during exercise, the changes of distensibility were much less in patients. Notwithstanding, of the 22 patients with quality echocardiographic data obtained during exercise, 18 (82%) had aortic distensibility below the lower limit of the 95% confidence interval of mean for controls at submaximal exercise.
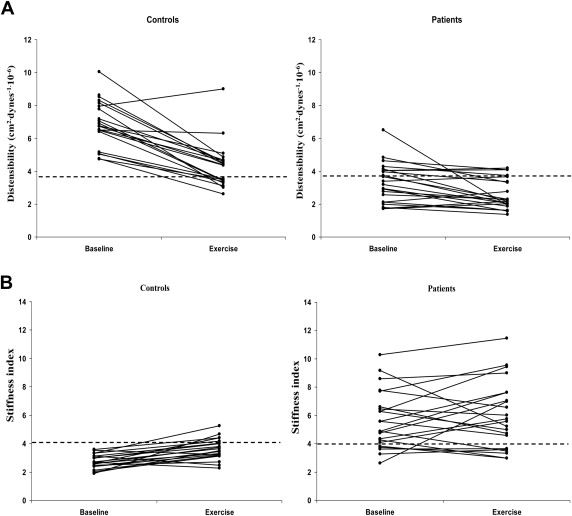
Changes of aortic stiffness with exercise are shown in Figure 1 . Whereas aortic stiffness was increased in most of the control subjects, the pattern was less consistent in patients. Furthermore, 73% (16 of the 22 patients with exercise data analyzed) had aortic stiffness greater than the upper limit of the 95% confidence interval of mean for controls at submaximal exercise.
Patients had significantly greater dimensions of the aortic annulus (24.2 ± 2.3 mm vs 18.8 ± 1.9 mm, p <0.001), sinus (35.6 ± 4.3 mm vs 25.0 ± 2.4 mm, p <0.001), sinotubular junction (27.1 ± 3.3 mm vs 19.3 ± 1.9 mm, p <0.001), and proximal ascending aorta (24.5 ± 3.9 mm vs 20.4 ± 2.4 mm, p <0.001) than controls. Normalized z scores at the 4 levels were 1.97 ± 0.86, 2.53 ± 0.87, 1.89 ± 1.30, and 0.69 ± 1.60, respectively; all except for the z score of ascending aortic dimension were significantly greater than zero (all p <0.05).
Of the 30 patients, 23 (76.7%) had significant dilation of aortic sinus ( z score >2). Patients with dilated aortic sinus compared with those without had significantly lower aortic distensibility, greater aortic stiffness, and higher cSBP at rest and lower aortic distensibility and strain at submaximal exercise ( Table 3 ). In contrast, demographic data, prevalence of associated intracardiac lesions, and history of pulmonary arterial banding were similar between these 2 groups of patients.
| Aortic Sinus Dilation | No Significant Aortic Sinus Dilation | p Value | |
|---|---|---|---|
| At rest | n = 23 | n = 7 | |
| Brachial-ankle pulse wave velocity (cm/s) | 1,043 ± 162 | 1,094 ± 286 | 0.56 |
| Femoral-ankle pulse wave velocity (cm/s) | 875 ± 149 | 822 ± 135 | 0.41 |
| Heart-femoral pulse wave velocity (cm/s) | 632 ± 62 | 663 ± 90 | 0.42 |
| Heart-carotid pulse wave velocity (cm/s) | 553 ± 94 | 557 ± 63 | 0.93 |
| cAI (%) | −25 ± 14 | −26 ± 11 | 0.90 |
| cSBP (mm Hg) | 112 ± 11 | 103 ± 7 | 0.039 ∗ |
| rAI (%) | 64 ± 14 | 60 ± 18 | 0.57 |
| rAI at 75 beats/min (%) | 63 ± 13 | 58 ± 17 | 0.41 |
| SBP (mm Hg) | 123 ± 14 | 114 ± 11 | 0.11 |
| DBP (mm Hg) | 66 ± 5 | 62 ± 6 | 0.07 |
| Aortic strain (%) | 12.2 ± 4.8 | 13.3 ± 2.5 | 0.43 |
| Aortic distensibility (cm 2 /dynes × 10 −6 ) | 3.2 ± 1.2 | 3.9 ± 0.5 | 0.035 ∗ |
| Aortic stiffness index | 5.9 ± 2.2 | 4.6 ± 0.6 | 0.023 ∗ |
| Carotid stiffness index | 5.5 ± 1.3 | 4.9 ± 0.5 | 0.21 |
| During exercise | n = 16 | n = 6 | |
| Aortic strain (%) | 13.1 ± 4.5 | 18.1 ± 4.9 | 0.033 ∗ |
| Aortic distensibility (cm 2 /dynes × 10 −6 ) | 2.3 ± 0.7 | 3.4 ± 1.0 | 0.01 ∗ |
| Aortic stiffness | 6.5 ± 2.4 | 4.8 ± 2.0 | 0.16 |
| Carotid stiffness † | 6.3 ± 2.4 | 6.0 ± 1.4 | 0.73 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


