Stratifying patients at a high risk for readmission and mortality before their discharge after acute decompensated heart failure (ADHF) is important. Although sleep-disordered breathing (SDB) is prevalent in patients with chronic heart failure, only few studies have investigated the impact of SDB on hospitalized patients with left ventricular (LV) systolic dysfunction after ADHF. Thus, we assessed the prevalence of SDB using nocturnal pulse oximetry and the relation between SDB and clinical events in this patient group. One hundred consecutive patients with LV systolic dysfunction who were hospitalized for ADHF were enrolled in the study. Predischarge nocturnal oximetry was performed to determine if they had SDB (defined as an oxygen desaturation index of ≥5 events/hour with ≥4% decrease in saturation level). Data on death and readmission for ADHF were collected. Forty-one patients had SDB. Complete outcome data were collected in the mean follow-up period of 14.2 months during which 33 events occurred. On multivariate Cox proportional hazards regression analysis, the presence of SDB was a significant independent predictor of postdischarge readmission and mortality (hazard ratio 2.93, p = 0.006). In conclusion, SDB, as determined by predischarge nocturnal oximetry, is prevalent and is an independent predictor of the combined end point of readmission and mortality in hospitalized patients with LV systolic dysfunction after ADHF.
Sleep-disordered breathing (SDB) is highly prevalent and is associated with increased morbidity and mortality in patients with chronic heart failure (HF) with left ventricular (LV) systolic dysfunction. A recent study demonstrated that in hospitalized patients with LV systolic dysfunction after acute decompensated HF (ADHF), coexisting SDB was significantly associated with an increased risk of 6-month cardiac readmission. However, performing fully equipped sleep studies in all such patients was not feasible; thus, nocturnal pulse oximetry was considered as an alternative. The presence of SDB, as determined by predischarge nocturnal pulse oximetry, might be associated with an increased postdischarge risk for readmission and mortality in patients with HF with LV systolic dysfunction after ADHF episodes.
Methods
One hundred consecutive patients who were hospitalized at the cardiology ward of Juntendo University Hospital with a diagnosis of decompensated HF from June 2000 to December 2002 were targeted for the present study. Decompensated HF was defined using modified Framingham criteria, including only those variables that were estimated at admission. Of these patients, only those with LV systolic dysfunction, defined as LV ejection fraction <50% on an echocardiogram, were prospectively enrolled. The exclusion criteria were acute coronary syndrome and cardiac surgery during the previous 4 weeks, end-stage renal disease requiring dialysis, cerebrovascular disease with neurological deficits, life-threatening malignancy, apparent obstructive lung disease as demonstrated by a forced expiratory volume <70% in 1 second/forced vital capacity, and known SDB. The study protocol was approved by the Juntendo University Hospital Institutional Review Board, and the study complied with the Declaration of Helsinki.
After the initial improvement of the acute signs and symptoms of HF within a few days before hospital discharge, nocturnal pulse oximetry was performed for 2 consecutive nights in the hospital using a finger pulse oximeter (Pulsox M24; Teijin Pharma, Tokyo, Japan); trained night-shift nurses noted the patients’ sleep time. We considered the recordings to be interpretable if they included ≥4 hours of observed sleep or if the signal loss was <1 hour. In patients without 2 interpretable recordings, we repeated the pulse oximetry assessment on the nights after the initial recordings. Arterial oxyhemoglobin saturation (SO 2 ) was recorded using a finger probe at a 1-Hz sampling frequency and a 5-second averaging time. These recordings were scored using specific software (DS-M; Teijin Pharma, Tokyo, Japan) with manual corrections performed by a trained scorer who was blinded to the baseline subject information. Mean SO 2 and the number of desaturations per hour of observed sleep, expressed as the oxygen desaturation index (ODI), were computed separately for each nightly recording, and then the 2 nights’ recordings were averaged. In the present study, desaturation was defined as ≥4% decrease in the saturation level and the presence of SDB was defined as ODI ≥5 events/hour. We chose these definitions because they were used in previous studies in which the impact of intermittent nocturnal oxygen desaturation in patients with HF was assessed.
On the same day as the pulse oximetry assessment, anthropometric data were obtained, and presence or absence of atrial fibrillation and the functional status of the patients were determined according to electrocardiography and New York Heart Association classification based on the specific activity scale, respectively. Blood pressure and heart rate were measured just before functional status assessment. Blood samples were obtained in the early morning after nocturnal oximetry was performed. Complete 2-dimensional echocardiography and Doppler ultrasound examination were performed using standard techniques on the day of blood sampling. These data were collected after the initial improvement of acute signs and symptoms of HF within a few days before hospital discharge.
After discharge, all patients were followed up in our hospital; in July 2003, outcome data were obtained by reviewing the medical records of our hospital in which all deaths and readmission events were recorded after discharge. The end point of the present study was a composite clinical outcome of death or readmission for ADHF. Readmission for ADHF was defined as the first unscheduled readmission to the cardiology ward with progressive symptomatic and/or hemodynamic deterioration.
All values are shown as the mean ± SD, and categorical variables are expressed as numbers and percentages. Baseline characteristics were compared using the Student t or Mann-Whitney U tests for continuous variables; the chi-square or Fisher’s exact tests were used for categorical variables. The survival curves were established using the Kaplan-Meier method and compared by the log-rank test. Univariate and multivariate Cox proportional hazards regression analyses were performed to evaluate the association between the presence of SDB and outcomes. On univariate analysis, the presence of SDB was used as an independent variable along with the following baseline variables: age; gender; body mass index; etiology of LV systolic dysfunction (i.e., ischemic or nonischemic); New York Heart Association class (i.e., class I/II or III/IV); presence or absence of atrial fibrillation; LV ejection fraction; blood pressure; heart rate; arterial partial pressure of oxygen and carbon dioxide (PaO 2 and PaCO 2 ); estimated glomerular filtration rate ; hemoglobin level; serum levels of sodium, potassium, and C-reactive protein; plasma B-type natriuretic peptide level; medication use; and mean SO 2 on nocturnal pulse oximetry. Variables that showed p <0.10 on univariate analysis were entered into a multivariate forward stepwise (p <0.05 for entering and excluding) Cox proportional hazards regression analysis. The assumption of proportional hazards was assessed using a log-minus-log survival graph. The best cut-off value of ODI for predicting outcomes was identified by receiver operating characteristic curve analysis at regular intervals as the value that minimized the expression [(1 − sensitivity) 2 + (1 − specificity) 2 ]. A value of p <0.05 was considered statistically significant.
Results
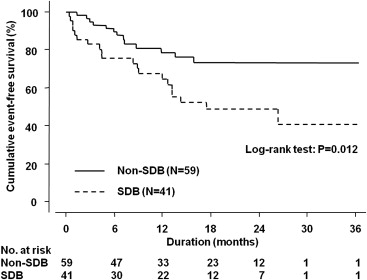
Of the 100 patients, 41 (41%) had SDB. The baseline characteristics of the patients with and without SDB, which were assessed before hospital discharge, are listed in Table 1 . The mean SO 2 and ODI are shown in Figure 1 .
| Variable | SDB | p Value | |
|---|---|---|---|
| No (n = 59) | Yes (n = 41) | ||
| Age (yrs) | 61 ± 11 | 64 ± 11 | 0.208 |
| Women | 13 (22) | 7 (17) | 0.722 |
| Body mass index (kg/m 2 ) | 21 ± 4 | 23 ± 4 | 0.007 |
| Ischemic etiology | 26 (44) | 14 (34) | 0.430 |
| New York Heart Association class III/IV | 17 (29) | 17 (41) | 0.272 |
| Diabetes mellitus | 17 (29) | 16 (39) | 0.394 |
| Atrial fibrillation | 9 (15) | 9 (22) | 0.553 |
| Systolic blood pressure (mm Hg) | 107 ± 14 | 109 ± 14 | 0.436 |
| Diastolic blood pressure (mm Hg) | 61 ± 8 | 61 ± 6 | 0.980 |
| Heart rate (beats/min) | 68 ± 9 | 71 ± 9 | 0.097 |
| LV ejection fraction (%) | 30 ± 10 | 28 ± 10 | 0.444 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 62 ± 19 | 54 ± 19 | 0.052 |
| Hemoglobin (g/dl) | 14 ± 2 | 14 ± 2 | 0.741 |
| Serum sodium (mmol/L) | 139 ± 4 | 140 ± 3 | 0.790 |
| Serum potassium (mmol/L) | 4.5 ± 0.4 | 4.4 ± 0.4 | 0.108 |
| Serum C-reactive protein (mg/dl) | 0.4 ± 0.1 | 0.8 ± 1.5 | 0.105 |
| Plasma B-type natriuretic peptide (pg/ml) | 147 ± 138 | 206 ± 206 | 0.101 |
| Partial pressure of arterial oxygen (Torr) | 93 ± 11 | 89 ± 14 | 0.113 |
| Partial pressure of arterial carbon dioxide (Torr) | 38 ± 4 | 39 ± 25 | 0.189 |
| Length of stay (days) | 16 ± 10 | 15 ± 10 | 0.804 |
| Medications | |||
| Angiotensin II receptor blockers/angiotensin-converting enzyme inhibitors | 46 (78) | 33 (80) | 0.956 |
| β Blockers | 24 (41) | 18 (44) | 0.748 |
| Loop diuretics | 43 (73) | 34 (83) | 0.240 |
| Aldosterone blockers | 28 (47) | 23 (56) | 0.395 |
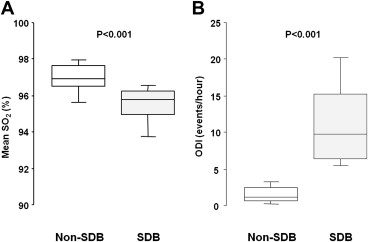
The outcome data were completely followed up for mean and maximum durations of 14 and 36 months, respectively. No patient was administered a specific therapy for SDB, such as positive airway pressure, use of an oral appliance, and supplemental oxygen, during follow-up because these therapies were not recommended for all patients with HF with LV systolic dysfunction at that time, and none of the patients complained of SDB-related symptoms or sought treatment. During this period, 33 clinical events (33%) occurred, of which 20 (49%) and 13 (22%) were encountered in the patients with and without SDB, respectively. The cumulative event-free survival curves of these 2 groups are shown in Figure 2 . On univariate Cox proportional hazards regression analysis, female gender (hazard ratio [HR] 3.22, 95% confidence interval [CI] 1.57 to 6.61, p = 0.002), lower body mass index (per 1-SD increment; HR, 0.71, 95% CI 0.48 to 1.04, p = 0.079), presence of atrial fibrillation (HR 2.21, 95% CI 1.05 to 4.66, p = 0.037), lower systolic blood pressure (per 1-SD increment; HR 0.72, 95% CI 0.49 to 1.05, p = 0.089), increased heart rate (per-1 SD increment; HR 1.38, 95% CI 1.03 to 1.86, p = 0.031), lower estimated glomerular filtration rate (per 1-SD increment; HR 0.57, 95% CI 0.39 to 0.83, p = 0.003), hemoglobin level (per 1-SD increment; HR 0.54, 95% CI 0.38 to 0.77, p <0.001), higher B-type natriuretic peptide level (per 1-SD increment; HR 1.68, 95% CI 1.24 to 2.29, p <0.001), use of loop diuretics (HR 11.4, 95% CI 1.55 to 83.1, p = 0.017), and the presence of SDB (HR 2.38, 95% CI 1.19 to 4.80, p = 0.015) showed values with p <0.1 and were included in the multivariable analysis. The final multivariate stepwise Cox proportional hazards regression analysis model is listed in Table 2 . The receiver operating characteristic curve analysis revealed that the best ODI cut-off value to predict clinical events was ≥5 events/hour (area under the curve 0.62 ± 0.06, p <0.045, sensitivity 61, specificity 72).