Fig. 53.1
A 2.9F Eagle Eye IVUS probe compatible with a 0.014-in. guidewire
Fig. 53.2
A motorized pullback sled device
The IVUS image created is a circular axial cut with a central disk artifact caused by the catheter. Real-time imaging displays the vessel wall and lumen at 30 frames/s allowing pulsatility of the artery wall to be seen. The ultrasound images display the vessel wall in histologic detail: the thin inner intima reflects the signal brightly while the media is a dark circumferential ring. Outside, the adventitia is also bright and reflective. Calcification typically reflects the ultrasound signal completely causing dark shadowing behind it (Fig. 53.3).
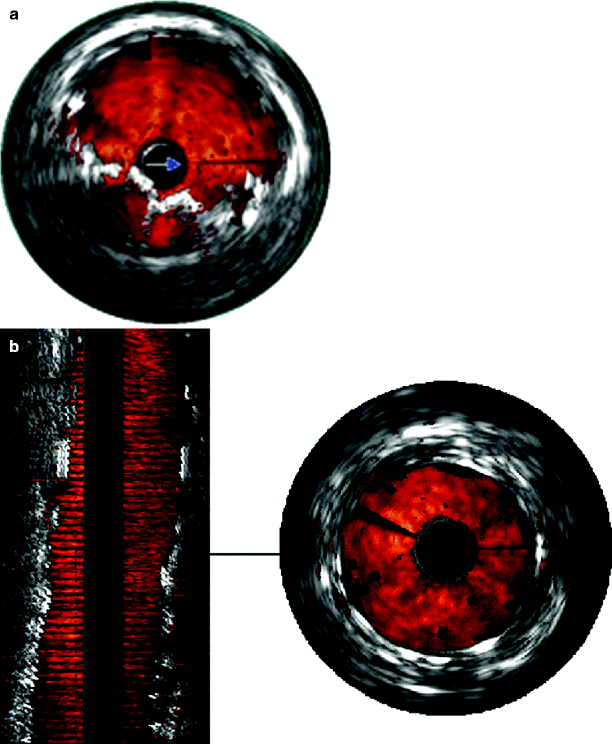

Fig. 53.3
(a) Two-dimensional color IVUS showing an incompletely deployed stent. (b) Two- and three-dimensional color IVUS showing improved stent deployment following reballooning
Three-dimensional reconstruction of the two-dimensional axial images produces a picture similar in appearance to an angiogram allowing the whole length of the vessel under examination to be viewed at the one time without the need for repeated pullbacks (Fig. 53.3). Consecutive axial images are stacked by powerful computing during a pullback of the ultrasound catheter through the vessel. A computerized edge tracking formula (algorithm) then aligns the consecutive frames [6]. The three-dimensional reconstruction can be viewed as either “longitudinal” or “volume” views. The longitudinal reconstruction is immediately available in the operating room and can be rotated around the longitudinal axis of the catheter to provide oblique and lateral perspectives. The volume view takes 2–3 min longer to create and has some artifact, since the raw data are altered. A smooth and steady pullback is necessary to acquire high-quality reconstructions.
Color flow IVUS was developed by EndoSonics (Rancho Cordova, CA, now Volcano Corporation). ChromaFlo is the computer software that detects blood flow and colors it red. The software detects the differences between adjacent IVUS frames. As the blood cells move through the artery, they move through the IVUS image frames. The software detects differences between adjacent frames and colors the image red. ChromaFlo detects faster flowing blood and colors it yellow. However, at present, flow velocities cannot be measured using this technique. ChromaFlo does not utilize the Doppler effect [5]. Color flow IVUS has been a very helpful addition because it shows clearly where the lumen meets the vessel wall. This was not always apparent in “black and white” IVUS, especially if the vessel wall contained dark echolucent plaque or soft thrombus. There are many clinical situations that color IVUS assists in but none more so than arterial dissection. A specific catheter, the Pioneer (Medtronic, MN), has been developed to identify true from false lumen and steer the guidewire (and treatment) accordingly.
Virtual histology IVUS (VH IVUS) is a new advance [11–14]. Where color IVUS has highlighted the blood flow in the lumen, VH IVUS aims to identify and color code plaque type in the artery wall. Conventional IVUS uses the amplitude of reflected ultrasound from the different components of the vessel and converts it into an image with a grayscale. VH IVUS analyzes the spectrum of reflected radiofrequency (RF) ultrasound signals and then classifies the spectral type into four categories of plaque:
1.
Fibrous tissue—densely packed collagen fibers with no evidence of lipid accumulation.
2.
Fibrofatty—loosely packed collagen fibers with regions of fatty deposit.
3.
Necrotic core—localized areas of loss of matrix containing lipid, typically with microcalcifications.
4.
Dense calcium—focal areas of dense calcium.
Each category is color coded (dark green—fibrous, light green—fibrofatty, red—necrotic core, white—calcification) and this is superimposed over the conventional grayscale IVUS image (Fig. 53.4). Comparison with coronary artery histologic examination has produced 80–93% accuracy rates [11].
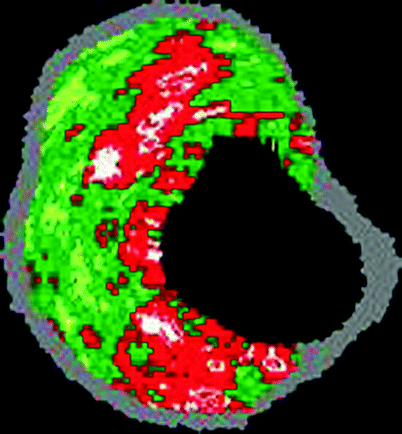

Fig. 53.4
Color-coded virtual histology IVUS of a calcified thin cap fibroatheromatous “vulnerable” plaque containing calcification (white), fibrous plaque (dark green), fibrofatty plaque (light green), and necrotic core plaque (red) (Courtesy of Volcano Corporation)
The clinical value of VH IVUS has yet to be defined, however, soft lipidic, necrotic core plaques have been implicated in acute ischemic syndromes such as plaque rupture. Detection of such “vulnerable” plaques using VH IVUS could have major treatment implications [10, 15]. While to date most work on VH IVUS has been in the coronary arteries, the peripheral arteries merit similar investigation, particularly the carotid arteries, since plaque rupture there can cause stroke. Treating such vulnerable plaque by stenting may 1 day become routine practice despite there being no hemodynamic stenosis if such vulnerable plaque is shown to be a high risk of stroke.
We have used VH IVUS most often in carotid artery stenting. We have found that such evaluation of carotid artery disease helps to anticipate how the plaque will behave at the moment of treatment. Will it resist complete stent expansion or break up and embolise to the brain? VH IVUS can only be performed in small caliber vessels using the Eagle Eye Catheter that has a range of 20 mm diameter.
Performing a Pullback
When the IVUS catheter has been chosen for the artery being treated (based on the size of the artery and the range of ultrasound that the catheter images), the probe is then flushed with heparinized saline and gently introduced over the wire. To obtain color flow, a “reference” function needs to be performed on the IVUS machine when the probe is in the arterial lumen. This removes near-field vision artifact, a characteristic of phased array IVUS imaging. The probe is passed into the artery beyond the disease and a slow and steady pullback is begun through the area in order to interrogate the lesion. Two- and three-dimensional views are then assessed.
Operative and Anatomic Situations
Carotid Angioplasty and Stenting
There are special features to bear in mind when stenting the carotid artery. It is particularly susceptible to incomplete stent deployment since heavy calcification resists complete stent expansion [16]. Furthermore, the internal carotid artery is not always uniform in diameter but is narrower distally and wider near its origin [17]. Stenting can also compromise the origin of the external carotid artery by compression, and recurrent stenosis following carotid endarterectomy may respond differently during stenting compared with a primary atherosclerotic lesion [18, 19]. The IVUS operator must appreciate these aspects and avoid dislodging embolic material or causing intimal dissection to the artery through overzealous instrumentation.
IVUS is a powerful tool in carotid stenting; however, the penalties are high if the disease is underestimated or the stent is badly deployed. We use IVUS more frequently now in carotid stenting since the introduction of cerebral protection devices. Immediately after deploying the protection device, IVUS can assess the lesion and take measurements to help stent and balloon sizing. We currently use the Eagle Eye Platinum catheter (Volcano Corporation, Rancho Cordova, CA), which is a low-profile 2.9F 20-MHz probe that configures with the 0.014-in. wire of most cerebral protection devices (Fig. 53.5).The IVUS probe is advanced into the distal internal carotid artery, and the pullback is begun. The distal internal carotid artery has a characteristic appearance on IVUS because it is so thin and usually spared of disease. The histologic layers are elegantly seen. Measurements can be taken just above the lesion to help size balloon and stent diameters, and the degree of stenosis can also be assessed to decide whether predilation is required or whether primary stent deployment can be performed [20]. While currently plaque morphology can be subjectively assessed by IVUS, the new advance of VH IVUS (Volcano Corporation, Rancho Cordova, CA) is promising since it will provide classified histologic plaque types.
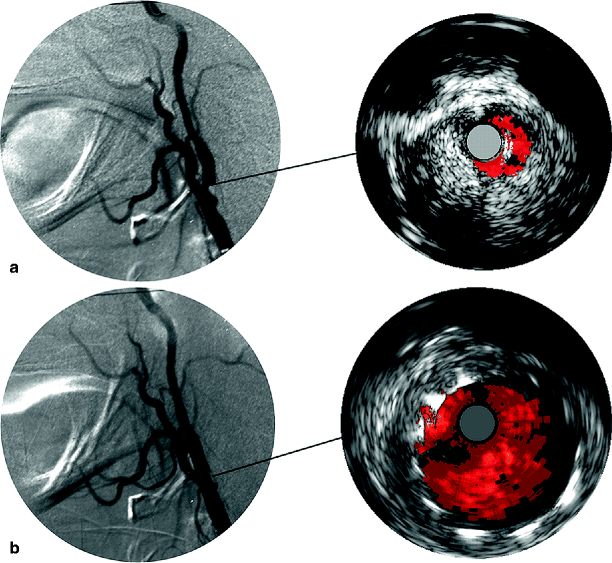

Fig. 53.5
(a) Intraoperative carotid angiogram and IVUS showing a stenosis of the internal carotid artery. (b) There is resolution of the stenosis poststenting. IVUS confirms excellent stent deployment
Following stenting, the IVUS probe is introduced again and a pullback assesses the completeness of stent deployment. IVUS measurements of the minimum stent diameter can be made to decide whether further ballooning is required. Mid-stent waisting is a common finding. In general, a minimum stent diameter of more than 4 mm is recommended in the internal carotid artery to avoid residual hemodynamically significant stenosis [7]. The stent should be uniformly well expanded throughout its length following deployment. It should also appose completely to the artery wall with no space apparent between the stent and the vessel. It is important to make sure that the length of the lesion is covered by the expanded stent such that no proximal or distal disease is left untreated. A careful inspection for intimal dissection is also important.
Despite our enthusiasm for carotid IVUS, passing balloon catheters and the IVUS probes up and down the internal carotid artery is not without hazard even with protection devices. The decision to reballoon or deploy another stent needs to be made cautiously. The advantages must be weighed against the risks of possible complication for the patient. Sometimes it is best to accept a reasonable deployment rather than overpursue it.
Stenting the origin of the common carotid artery from a retrograde carotid approach poses a challenge to all endovascular specialists. The common carotid artery comes off the aortic arch or the innominate artery, and accurate stent location at the origin is difficult using aortography alone because of the natural curvature of the arch [20] (Fig. 53.6). Accurate placement is possible using IVUS by passing the IVUS probe down the common carotid artery from above. Fluoroscopy visualizes the radiopaque transducer as it passes down the vessel. Blood flow is displayed in color pulsing red with each cardiac cycle. When the probe passes from the stenosis into the aorta, there is a sudden change in the size of the luminal color. Together with fluoroscopy, this technique pinpoints the origin of the vessel exactly for accurate device deployment. The same technique can also be used to stent the origin of all the supraaortic vessels.
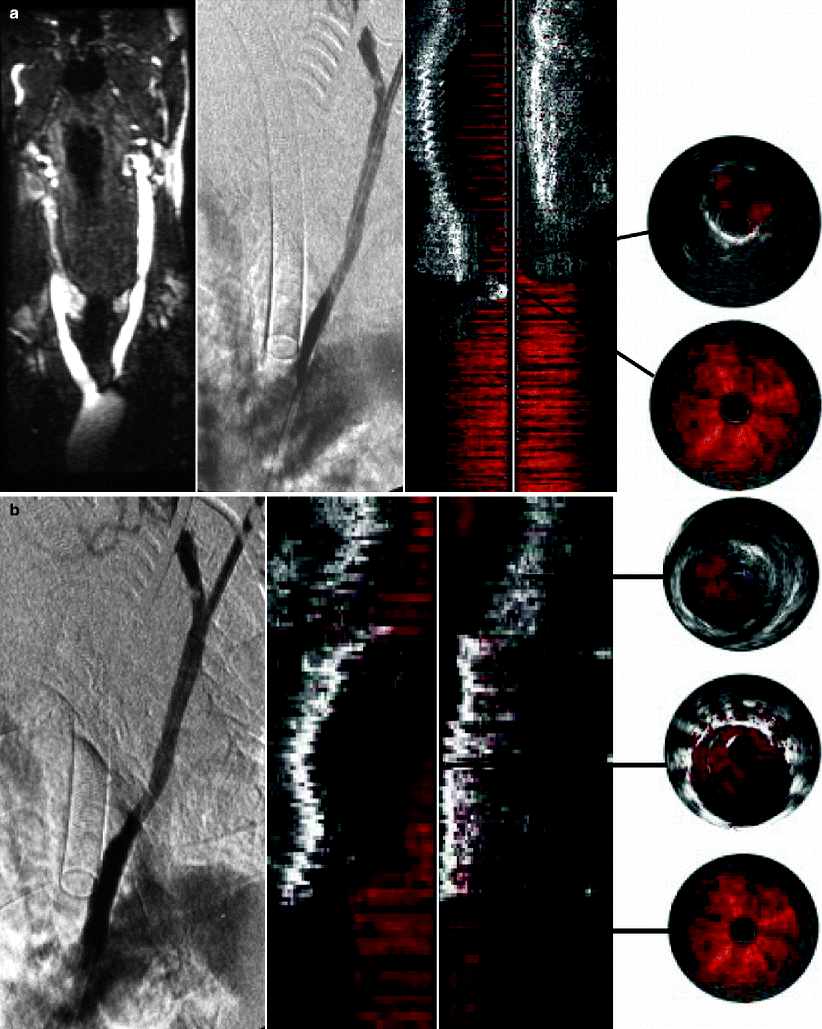

Fig. 53.6




(a) Magnetic resonance angiography showing a tight stenosis at the origin of the left common carotid artery. At operation, the IVUS probe is passed down from a cervical access assisting road mapping. Two- and three-dimensional color IVUS locates the origin of the carotid artery. (b) Two- and three-dimensional color IVUS showing accurate stenting at the origin of the common carotid artery together with the completion angiogram (Reprinted from Irshad et al. [20]. With permission from Informa Healthcare)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


