Arrhythmias in Congenital Heart Disease
George F. Van Hare
Patients with congenital heart disease are challenging for many reasons. When such patients develop arrhythmias, as they often do, their arrhythmias are often difficult to adequately control. Such problems are often quite disappointing to the patients and their families because many such patients have had a successful repair with a good hemodynamic result but nonetheless continue to require medical interventions.
There are a number of arrhythmia mechanisms in patients with congenital heart disease. Although the great majority of such arrhythmias are seen in postoperative patients, certain other patients who have not undergone surgery are also at some increased risk. The principles of management, however, are similar for both groups. This chapter describes the causes for arrhythmias in both the postoperative and preoperative state and discusses some of the treatment strategies that are available.
Arrhythmias after Congenital Heart Disease Repair
When a child is born with congenital heart disease and requires surgical repair of the heart defect, most of the focus of the surgeon, cardiologist, and parents is on obtaining as good a hemodynamic result as possible. For many defects, such as atrial or ventricular septal defects, atrioventricular canal defects, and tetralogy of Fallot, surgical results are excellent and one can expect to have a perfect or nearly perfect hemodynamic result. With more complex defects, such as transposition of the great arteries, surgery can also be expected to produce a normal or nearly normal hemodynamic situation with an excellent long-term prognosis for the child. In even the most complex defects, including the single-ventricle lesions, good results can also be obtained, prolonging life into adulthood and further. It is in this setting that one must consider the impact of late postoperative arrhythmias. These arrhythmias can be annoying, debilitating, or even life threatening.
Because late postoperative arrhythmias contribute significantly to morbidity and mortality, it is logical that every effort is made in patients with such arrhythmias to treat them and prevent their recurrence. Such treatments have included medical therapy with antiarrhythmic agents, implantation of antitachycardia pacemakers, catheter ablation, and surgical ablation. Unfortunately, there are serious limitations to the effectiveness, applicability, and safety of both antiarrhythmic drug therapy and antitachycardia pacing. Not surprisingly, ablative techniques, which potentially offer a curative treatment, are favored, but such techniques have not been as successful as initially hoped.
Principal Patient Groups
Postoperative tachyarrhythmias tend to group themselves into four main groups based on type of defect, type of tachycardia, and current success rates of radiofrequency ablation attempts. The first group, referred to as simple atriotomy-based atrial flutter, includes patients who have had simple cardiac repairs such as atrial septal defects, ventricular septal defects, tetralogy of Fallot, atrioventricular canal defects, and related defects. The second group, termed intraatrial reentry after atrial repair of transposition (IART), includes patients who have had either the Mustard or the Senning procedure. The third group, termed intraatrial reentry after the Fontan procedure, includes patients who have undergone the various forms of the Fontan procedure. Finally, a fourth group, termed ventricular tachycardia
after tetralogy repair, includes tetralogy of Fallot patients, as well as those with related lesions such as certain types of double-outlet right ventricle. The anatomic details that support the tachyarrhythmia and are important in ablation are discussed for each group.
after tetralogy repair, includes tetralogy of Fallot patients, as well as those with related lesions such as certain types of double-outlet right ventricle. The anatomic details that support the tachyarrhythmia and are important in ablation are discussed for each group.
Anatomic and Developmental Considerations
Because atrial flutter and nearly all types of postoperative atrial tachycardia seem to involve only the anatomic right atrium, the exact anatomy of the right atrium becomes important in understanding these arrhythmias. Therefore, a detailed knowledge of right atrial anatomy is essential for effective mapping and ablation of IART and atrial flutter.
During cardiac embryologic development, the right atrium is thought to be derived from three sources (1). The primitive right atrium forms adjacent to the tricuspid annulus and gives rise to the heavily trabeculated right atrial free wall and right atrial appendage. The sinus venosus is incorporated into the right atrium and provides the origin for the smooth-walled portion of the right atrium (sinus venarum) that exists between the cavae posterior to the primitive right atrial structures. All along the junction between the primitive right atrium and the sinus venosus portion of the right atrium is the crista terminalis, which appears as a ridge along the inner surface of the right atrium. The crista terminalis runs from superior to inferior along the lateral wall of the right atrium. At its superior edge, near the superior vena cava–right atrial junction, is the sinus node pacemaker complex. As it arches inferiorly toward the inferior vena cava, it gives rise to the Eustachian valve ridge (EVR), which appears as more of a flap than a ridge. The EVR runs anterior to the inferior vena caval orifice and posterior to the posterior portion of the tricuspid valve annulus. As such, in the fetal circulation, the EVR acts to direct inferior vena caval (IVC) flow away from the tricuspid annulus and toward the foramen ovale. As the EVR arches toward the inferior atrial septum, it passes just superior to the ostium of the coronary sinus. It joins with the valve of the coronary sinus to form the tendon of Todaro, which inserts on the atrial septum near to where the His bundle is recorded.
“Adult-Type” Atrial Flutter
Classic atrial flutter is characterized by atrial rates of approximately 300 beats per minute with typical and very characteristic “saw tooth” flutter waves visible on the surface electrocardiogram. This suggests the presence of nearly continuous atrial activity due to the relative lack of a long atrial isoelectric interval. In typical atrial flutter, the flutter waves are prominent and are negative in leads II, III and AVF, suggesting inferior-to-superior atrial activation. The work of multiple investigators has clearly established the flutter circuit. Impulses emerge from an isthmus of atrial tissue between the inferior vena cava and tricuspid annulus to spread up the atrial septum, activating the atrium at the site of the His bundle recording, and then down the right atrial free wall to enter the isthmus again (2). This counterclockwise activation is categorized as “typical” atrial flutter, whereas atrial flutter that uses the same circuit but in the clockwise order of activation is categorized as “reverse typical” atrial flutter (3). In addition, further details have been provided using techniques of entrainment pacing that depend on the demonstration of equivalence of the postpacing interval (PPI) during entrainment and the tachycardia cycle length (TCL) to establish that any given site is in the circuit (PPI = TCL). These studies have demonstrated the importance of the crista terminalis and EVR as sites of conduction block during atrial flutter (4,5,6). Conduction block is suggested by the demonstration that there are sites along the ridge where double potentials can be recorded (4,5,7,8). The importance of such areas of conduction block is confirmed by entrainment pacing, demonstrating that atrial myocardium on one or the other side of the line of conduction block is part of the circuit. The critical nature of these lines of block is proven by radiofrequency ablation lesions that are designed to bridge from one line of block to another, with resultant abolition of the atrial flutter (9,10,11). These criteria have been satisfied with both typical and reverse typical atrial flutter, and the features of this arrhythmia circuit now seem well characterized.
As previously described, the wave of activation leaves the region of the tricuspid valve–inferior vena cava isthmus to climb the interatrial septum and enter the heavily trabeculated right atrial free wall in the region of the superior vena cava. It then spreads down the right atrial free wall, with the crista terminalis behind and the tricuspid annulus in front, turning counterclockwise around the tricuspid annulus when viewed from below (left anterior oblique view fluoroscopically). As the wave of activation turns to the posterior, it approaches the isthmus between the inferior vena cava and tricuspid valve annulus, now with the annulus anterior and inferior, and the EVR (the extension of the crista terminalis) posterior and superior. It is important to note that at this site, the EVR bisects the isthmus between the IVC and tricuspid valve, and that it is the EVR, not the IVC, that provides the critical site of conduction block. As it enters the interatrial septum, the wave again spreads in a superior fashion along the septum for the next circuit. Atrial flutter can be effectively attacked using radiofrequency ablation either at the septal site of EVR insertion, by lesions that bridge from the tricuspid valve to the EVR (5), or at a posterior location, from the tricuspid annulus down to the IVC (11).
Simple Atriotomy-Based Atrial Flutter
When performing surgery to repair a simple secundum atrial septal defect, the surgeon typically places a long incision in the right atrial free wall, which is oblique and runs from the right atrial appendage laterally down toward but not to the tricuspid annulus or IVC. This incision gives adequate exposure for repair of atrial septal defects and is also used for the atrial approach to repair of ventricular septal defects, either alone or as part of tetralogy of Fallot. The atrial septal defect itself is commonly closed using sutures, but a patch may be employed for large defects.
This surgical approach clearly creates a long line of permanent conduction block that is entirely in the trabeculated right atrium, anterior to the crista terminalis. This anatomy potentially creates a tunnel of atrial tissue between the crista and the atriotomy and another between the atriotomy and the tricuspid annulus. Such tunnels can easily be imagined as the required protected zones of conduction, supporting IART. Numerous patients have now been reported who exhibit “incisional” IART in which the atriotomy seems to act as the critical barrier (4), and in such patients, radiofrequency application from the atriotomy to either the IVC, tricuspid annulus, or superior vena cava (SVC) has been successful in terminating tachycardia and preventing reinduction.
Because the atrial structures that support typical atrial flutter are also present, and because such patients often have other risk factors for the development of flutter (atrial dilation, fibrosis, etc.), postoperative patients may also have typical atrial flutter. Furthermore, as has become apparent in patients with otherwise structurally normal hearts, such IART and flutter circuits can run in either direction (counterclockwise or clockwise). Indeed, several series have reported a more common occurrence of typical or reverse typical atrial flutter than true incisional flutter in such patients (12,13). Finally, in patients
who have undergone patch closure of a secundum atrial septal defect, the patch itself has been reported to be a possible site of conduction block, mediating tachycardia (11), although this is much less common in practice. The potential variability in circuits and rotation creates the possibility for several distinct P-wave morphologies and atrial cycle tachycardia cycle lengths.
who have undergone patch closure of a secundum atrial septal defect, the patch itself has been reported to be a possible site of conduction block, mediating tachycardia (11), although this is much less common in practice. The potential variability in circuits and rotation creates the possibility for several distinct P-wave morphologies and atrial cycle tachycardia cycle lengths.
Patients may, of course, have several reentrant circuits. One commonly observes typical atrial flutter and, after successful ablation, a second IART with a longer or shorter tachycardia cycle length and noninvolvement of the flutter isthmus, or indeed of any structure posterior to the crista terminalis. The slower cycle length of such atrial tachycardias may be due to conduction that is confined to the heavily trabeculated atrial free wall, in which atrial conduction may be slower, longer circuits due to dilation or may reflect slow conduction due to fibrosis.
Intraatrial Reentry after Atrial Repair of Transposition
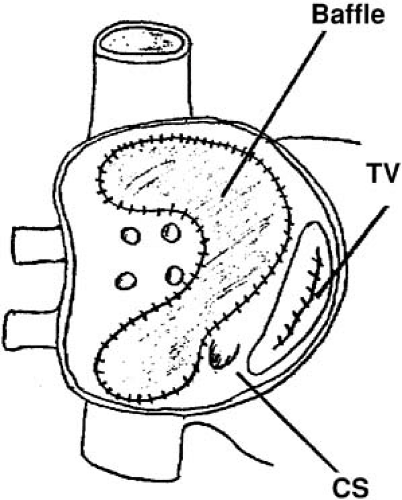
The Senning and Mustard procedures are similar operations meant to address the hemodynamic abnormality in transposition by directing systemic return to the left ventricle and pulmonary artery and directing pulmonary venous return to the right ventricle and aorta (14,15). Although they are very successful, these operations are for the most part no longer done, in part because of the success of the arterial switch procedure and in part due to the high incidence of sinus node dysfunction, atrial arrhythmias, and increased risk of sudden death. However, there has recently been interest in the so-called “double-switch” procedure as a strategy for managing patients with L-transposition (congenitally corrected transposition), and in the procedure, a Senning atrial baffle is constructed (16). Therefore, this surgical substrate may not, in fact, disappear in the future. In the Mustard procedure, after a long atriotomy anterior to the crista terminalis and resection of the atrial septum, a baffle is constructed and sewn into place around each caval vein, through the isthmus between the IVC and tricuspid annulus, and to the posterior wall of the left atrium, so that caval flow is direct to the mitral annulus (14). Pulmonary venous flow travels around the baffle and is directed to the tricuspid annulus (Fig. 70.1). Furthermore, surgical technique is directed at avoiding injury to the sinus node, so the crista terminalis is not disturbed. Finally, various approaches are used to avoid atrioventricular block, and often these lead to the coronary sinus being incorporated into the pulmonary rather than systemic venous atrium (17). These details leave the entire right atriotomy as well as the isthmus of atrial tissue between the EVR and tricuspid annulus in the new pulmonary venous atrium (18,19). The one exception is the situation in which the coronary sinus drainage is the systemic venous atrium, in which an ablation catheter can reach the flutter isthmus from the IVC (19).
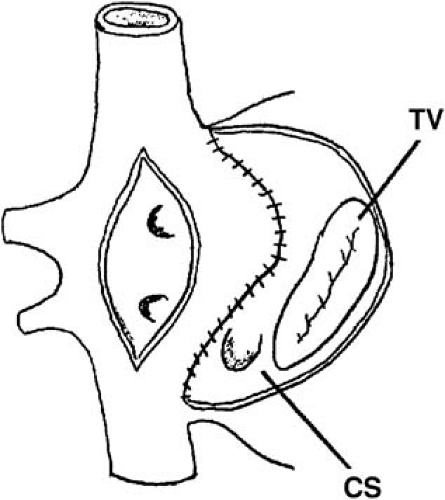
In most respects, the Senning procedure is similar electrophysiologically to the Mustard procedure. The Senning procedure was designed to use mostly atrial tissue rather than artificial material to construct the baffle (15). To accomplish this, two atrial incisions are made. The first is in the right atrium, longitudinal, parallel, and anterior to the crista terminalis. The second, in the left atrium, is parallel to the first and between the right pulmonary veins and interatrial septum. A U-shaped incision in the atrial septum is made just above the coronary sinus, leaving the flutter isthmus intact. This flap of atrial septum is sewn to the back of the left atrium, to the left of the left pulmonary veins (Fig. 70.2). The flap of right atrial free wall is sewn into place near or at the site of the EVR, preventing IVC flow from crossing the tricuspid valve. The left atrial incision is closed by sewing to the other edge of the right atrial incision. As in the Mustard procedure, both the flutter isthmus and the right atriotomy are part of the new pulmonary venous atrium.
Typically, and predictably based on the foregoing, one commonly finds two types of IART in such patients. First, typical or reverse typical atrial flutter is usually present, using
the usual anatomic structures as barriers to support reentry. Second, true “incisional” IART is often found and is confined to the anatomic trabeculated right atrium, with the wave of activation passing between the lower limit of the atriotomy and the tricuspid annulus.
the usual anatomic structures as barriers to support reentry. Second, true “incisional” IART is often found and is confined to the anatomic trabeculated right atrium, with the wave of activation passing between the lower limit of the atriotomy and the tricuspid annulus.
Intraatrial Reentry after the Fontan Procedure
The Fontan procedure has changed many times since its development as a palliative procedure for patients without two functional ventricles, as a way of relieving ventricular volume overload and of normalizing arterial saturations (20). Initially, it was thought that the right atrium could be used as an effective pumping chamber, provided that pulmonary artery pressures were low (atriopulmonary connection). Largely as a result of an extremely high incidence of atrial arrhythmias after such procedures as well as concerns about hydraulic energy loss in the system and pulmonary venous obstruction, this approach has been abandoned in favor of approaches that bypass the heart entirely (total cavopulmonary connection via the lateral tunnel or via an external conduit) (21,22,23). Many modifications exist within each of the two categories. Despite the approach of total cavopulmonary connection, atrial arrhythmias continue to be observed, and surgical details are critical in planning mapping and ablation procedures in such patients.
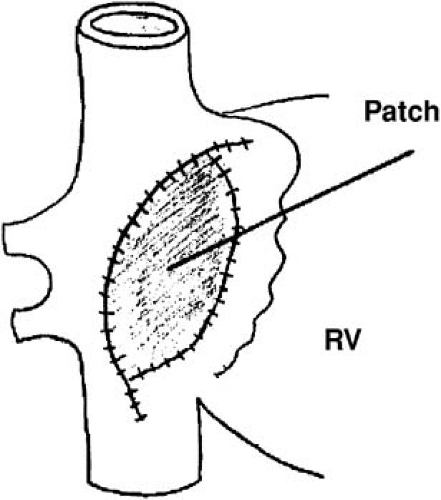
A long atriotomy was placed in the various forms of atriopulmonary connection. In patients who had a conduit from the right atrium to pulmonary artery and those in whom the right atrial appendage was connected directly to the pulmonary artery or right ventricular outflow tract, this atriotomy was anterior to the crista terminalis. Often in such patients, patch augmentation of the right atrium was performed, using a piece of pericardium or other material incorporated into the closure (Fig. 70.3). Invariably, closure of a large atrial septal defect was also necessary. As in the simpler situation of atrial septal defect (ASD) repair (see prior discussion), both typical atrial flutter and incisional reentry around the anterior atriotomy are possible and have been observed. In excellent multisite mapping studies using basket catheters, Triedman et al. demonstrated slow conduction up the lateral wall (24), and this configuration fits the concept of conduction in a long isthmus bounded by the atriotomy and the crista terminalis. Reentry around the ASD patch is also possible. Finally, patch closure of the tricuspid annulus has been occasionally performed in patients with single ventricle without tricuspid atresia, potentially creating areas of slow atrial conduction on the other side of the suture line.
Ventricular Tachycardia after Tetralogy Repair
Ventricular tachycardia continues to be a difficult management problem in patients who have previously undergone surgical repair of tetralogy of Fallot and other related congenital heart defects. The etiology of sudden death in patients after tetralogy repair is somewhat uncertain. However, because of the frequent occurrence of premature ventricular contractions, nonsustained and sustained ventricular in patients who have undergone complete repair of tetralogy of Fallot and related defects such as double-outlet right ventricle (25,26,27,28,29,30,31), ventricular tachycardia has been implicated in the etiology of sudden death in this patient group. Indeed, it is known that postoperative tetralogy of Fallot is the most common condition seen in children between the ages of 1 and 16 years who experienced sudden death (32).
Most of the information concerning ventricular tachycardia in congenital heart disease pertains to tetralogy of Fallot as compared with other forms of congenital heart disease. Ventricular arrhythmias do occur but are much more rare in patients with other lesions (33). However, for the purposes of management, tetralogy of Fallot can be viewed as a model for other lesions when patients with other lesions present with ventricular arrhythmias in the setting of ventriculotomy and/or right ventricular dysfunction.
Some controversy exists regarding the role of various risk factors for the occurrence of ventricular arrhythmias and sudden death, the exact relationship between ventricular arrhythmias and sudden death, the role of electrophysiology study and other procedures for risk stratification, and ultimately the appropriate management of postoperative patients with ventricular tachycardia.
Patients with tetralogy of Fallot before repair have a ventricular septal defect with (usually severe) right ventricular outflow tract obstruction, leading to chronic cyanosis. The placement of a systemic-to-pulmonary artery shunt as a palliative procedure adds the element of chronic left ventricular volume overload. Correction of the defect involves patch closure of the ventricular septal defect with relief of right ventricular
obstruction. In nearly all patients, this requires resection of a large amount of right ventricular muscle, and early in the experience, this was not done via an atriotomy with retraction of the tricuspid valve, but instead required a ventriculotomy. Finally, in tetralogy, the pulmonary annulus is typically smaller than normal. This has been approached by the placement of a transannular patch, which leads to chronic pulmonic insufficiency. Pulmonic insufficiency may be very severe if it is associated with downstream obstruction related to significant pulmonary arterial stenosis. It has been hypothesized that ventricular arrhythmias are due to the effect of years of chronic cyanosis, followed by the placement of a ventriculotomy, with elevation of right ventricular pressures due to inadequate relief of obstruction, and severe pulmonic regurgitation with right ventricular dysfunction and enlargement (27,34,35,36). Such factors as wall stress and chronic cyanosis, coupled with the passage of time, may lead to myocardial fibrosis and result in the substrate for reentrant ventricular arrhythmias. This hypothesis is supported by histologic examination of the hearts of patients with tetralogy of Fallot who died suddenly. These studies have shown extensive fibrosis (37). The hypothesis is also supported by the observation of fractionated electrograms and late potentials recorded from the right ventricle at electrophysiology study, suggesting the presence of slow conduction (38,39).
obstruction. In nearly all patients, this requires resection of a large amount of right ventricular muscle, and early in the experience, this was not done via an atriotomy with retraction of the tricuspid valve, but instead required a ventriculotomy. Finally, in tetralogy, the pulmonary annulus is typically smaller than normal. This has been approached by the placement of a transannular patch, which leads to chronic pulmonic insufficiency. Pulmonic insufficiency may be very severe if it is associated with downstream obstruction related to significant pulmonary arterial stenosis. It has been hypothesized that ventricular arrhythmias are due to the effect of years of chronic cyanosis, followed by the placement of a ventriculotomy, with elevation of right ventricular pressures due to inadequate relief of obstruction, and severe pulmonic regurgitation with right ventricular dysfunction and enlargement (27,34,35,36). Such factors as wall stress and chronic cyanosis, coupled with the passage of time, may lead to myocardial fibrosis and result in the substrate for reentrant ventricular arrhythmias. This hypothesis is supported by histologic examination of the hearts of patients with tetralogy of Fallot who died suddenly. These studies have shown extensive fibrosis (37). The hypothesis is also supported by the observation of fractionated electrograms and late potentials recorded from the right ventricle at electrophysiology study, suggesting the presence of slow conduction (38,39).
Careful electrophysiology studies in patients with ventricular tachycardia after tetralogy surgery have supported the concept that the mechanism of ventricular tachycardia is macroreentry, which involves the right ventricular outflow tract either at the site of anterior right ventriculotomy or at the site of a ventricular septal defect patch. Transient entrainment has been documented, with constant fusion at the paced cycle length and progressive fusion at decreasing cycle lengths. Furthermore, the evaluation of postpacing intervals strongly suggests that sites in the right ventricular outflow tract are part of a macroreentrant circuit (40,41).
Early reports noted the frequent occurrence of premature ventricular contractions in patients who had previously undergone tetralogy of Fallot repair. Gillette et al. identified premature ventricular contractions on routine electrocardiograms in 18% of patients (27). With exercise testing, the incidence may increase, to around 20% in one study (26). With Holter monitoring, the incidence of ventricular ectopy is reported to be as high as 48% (42). In about half of these patients, ventricular ectopy is complex, defined as multiform beats, couplets, or ventricular tachycardia. In the great majority of patients, this ventricular ectopy is entirely asymptomatic.
Many investigators have tried to correlate the incidence of ventricular ectopy with various factors, including age at presentation, age at time of repair, and various hemodynamic features. Four factors seem to be the most important: (a) age at initial repair, (b) time since repair, (c) presence of residual right ventricular obstruction, and (d) presence of significant pulmonic insufficiency. Older age at time of operation, especially beyond 10 years of age, was associated with nearly a 100% incidence of ventricular arrhythmias, regardless of follow-up interval, in a multicenter study (42). In the same study, time since repair also predicted the occurrence of ventricular ectopy, which occurred in 4 of 4 patients followed for more than 16 years, despite repair in infancy. Walsh et al., however, showed that in a group of patients repaired at less than 18 months of age, ventricular ectopy was rare on electrocardiogram (1%) but more common on Holter (31%) after an average of 5 years of follow-up (43).
Garson et al., in a study of 488 patients with repaired tetralogy of Fallot, showed that the incidence of ventricular arrhythmias was closely related to right ventricular hemodynamics (44). The incidence of ventricular arrhythmias was significantly higher in those with a right ventricular systolic pressure greater than 60 mm Hg and in those with a right ventricular end-diastolic pressure greater than 8 mm Hg, suggesting that residual right ventricular outflow tract obstruction as well as pulmonic insufficiency negatively influence outcome. They also found a relationship to age at surgery, but this was not as important as the follow-up interval. Zahka et al., in a prospective study of 59 patients with tetralogy of Fallot repaired prior to 11 years of age, found that the degree of pulmonary regurgitation was by far the most important predictor of the frequency and severity of spontaneously occurring ventricular arrhythmias (36). Although the degree of residual right ventricular outflow tract obstruction was not a predictor in this study, significant residual obstruction was rare in their study group.
Spontaneously occurring sustained ventricular tachycardia is, in fact, fairly uncommon among patients with repaired tetralogy, in distinction to the high incidence of ventricular ectopy. The best data in this regard come from a report by Harrison et al. of patients with repaired tetralogy of Fallot attending an adult congenital heart disease clinic (45). Eighteen of 210 patients (8.6%) had either documented sustained ventricular tachycardia, syncope, or near-syncope with palpitations and inducible sustained monomorphic ventricular tachycardia at electrophysiology study. Ventricular tachycardia was closely related to right ventricular hemodynamics and, in particular, right ventricular outflow tract aneurysms and pulmonic insufficiency. This finding is consistent with the earlier report by Zahka et al. (36) that emphasized the importance of pulmonic insufficiency as a risk factor for ventricular ectopy.
The occasional but persistent observation of sudden, unexpected death in this group of patients with repaired congenital heart disease, along with the high incidence of spontaneously occurring ventricular arrhythmias, both simple and complex, has led to the hypothesis that sudden death in such patients is due to ventricular tachycardia. During the 1970s and 1980s, it was the standard practice to perform electrophysiology studies with programmed stimulation in a large proportion of patients who had undergone repair of tetralogy of Fallot or related congenital defects (Fig. 70.4). Antiarrhythmic drug therapy was often prescribed based on the results of such studies. This approach has, for the most part, been abandoned due to the lack of strong evidence supporting the proposition that sudden death can be prevented with this approach, as well as worries about the proarrhythmic effect of the antiarrhythmic medications chosen for treatment. In a large multicenter review, Chandar et al. reported experience with 359 postoperative tetralogy of Fallot patients who underwent invasive electrophysiology testing (42). Ventricular tachycardia could be induced in 17% of patients but not in any patient who was asymptomatic and had a normal 24-hour electrocardiogram. Although late sudden death occurred in 5 patients, none of these patients had inducible ventricular tachycardia at electrophysiology study.
One can assess the risk of ventricular tachycardia from the surface electrocardiogram QRS duration. Gatzoulis et al. found that in a group of 48 well-studied postoperative tetralogy patients, those with a QRS duration of 180 msec or greater had a greatly increased risk of spontaneous ventricular tachycardia and/or sudden death (35). Similarly, Balaji et al. showed that a QRS duration of 180 msec or greater predicts the finding of inducible sustained monomorphic ventricular tachycardia at electrophysiology study (46). The cause of this relationship is uncertain.
There is some evidence that the risk of sudden death may increase at late follow-up. In a careful study of 490 survivors of tetralogy of Fallot repair at a single center, Nollert et al. constructed actuarial survival curves out to 36 years after surgery (47). In this study, the yearly actuarial mortality rate during the first 25 years was 0.24%/year, but mortality increased dramatically after 25 years to 0.94%/year. Most mortality was due to sudden death. The mortality risk was also related to date of operation (highest before 1970), the degree of preoperative polycythemia (highest with hematocrit >48), and the use of a right ventricular outflow tract patch (highest with a patch). The last factor is most likely related to the presence of pulmonic insufficiency, as suggested earlier.
Surgically Induced Atrioventricular Block
The risk of complete atrioventricular (AV) block as a result of surgery depends on the type of repair that is attempted. At
highest risk are patients who undergo closure of atrioventricular septal (canal or endocardial cushion) defects and those having closure of ventricular septal defects involving the perimembranous region of the ventricular septum (33



highest risk are patients who undergo closure of atrioventricular septal (canal or endocardial cushion) defects and those having closure of ventricular septal defects involving the perimembranous region of the ventricular septum (33
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree