CHAPTER 132 Arrhythmia and Pacemaker Surgery in Congenital Heart Disease
The progress in the management of cardiac arrhythmias related to congenital heart disease over the past 40 years mirrors the improvement in outcomes associated with heart disease in children. During the earliest era in the management of congenital heart disease, postoperative arrhythmias and heart block were a substantial cause of perioperative mortality. With the advent of direct myocardial pacing and cardiac defibrillation more than 50 years ago, mortality rates dropped substantially. In fact, the earliest history of pacing dates back to the repair of congenital heart disease. In 1958, Brockman and colleagues1 were the first to report the use of myocardial pacing electrode after repair of a ventricular septal defect. Subsequent advances in arrhythmia detection and therapy coupled with the remarkable progress in pacemaker and defibrillator technology have resulted in a significant evolution in the field of arrhythmia therapy. As a result of the miniaturization of technology and the advances in transvenous techniques, cardiac surgeons have been displaced by the cardiologist in most primary electrophysiologic interventions, both diagnostic and therapeutic. This is especially true in the domain of adult cardiac surgery, where most ablative and pacemaker procedures have been transformed into a transvenous approach performed in the laboratory by cardiologists. Although the same is largely true in treatment of pediatric arrhythmias and arrhythmias associated with congenital heart disease, the inherent size and anatomic constraints in those patients often require more surgical participation in the arrhythmia therapy.
INDICATIONS AND TECHNIQUES FOR ELECTROPHYSIOLOGIC DEVICE THERAPY IN PEDIATRIC AND CONGENITAL HEART DISEASE
Pacemakers
The recommendations for permanent pacing in children and patients with congenital heart disease are updated regularly. The last update was a practice guideline published in 2002 jointly by the American Heart Association (AHA), the American College of Cardiology (ACC), and the Heart Rhythm Society (HRS), and its recommendations are listed in Box 132-1.2 A class I indication for pacing exists for atrioventricular (AV) block that persists for more than 7 days after cardiac surgery. (The 7-day cutoff is the starting point, with longer waits for very small or unstable patients who have adequate backup temporary pacing.) The recommendations put special emphasis on age-appropriate heart rates and the presence of CHD or ventricular dysfunction to guide treatment decisions.
Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Source: American Heart Association, Inc.
Class I
Class IIa
Class IIb
Additional indications for permanent pacing may not be as obvious as those listed in the AHA/ACC/HRS guidelines. If a child with a congenital heart defect requires surgery, it may be prudent to preemptively implant a pulse generator or epicardial leads during the surgery. This may benefit children with preoperative rhythm abnormalities who do not meet the indications listed in Box 132-1, but for whom pacing will be needed postoperatively based on the known natural history of a particular cardiac anomaly or type of surgery. For example, an infant with L-transposition of the great arteries undergoing ventricular septal defect closure is at risk for complete AV block even without cardiac surgery and may benefit from prophylactic epicardial lead placement. Such placement may also assist children undergoing Fontan revision surgery in conjunction with an atrial maze procedure that is likely to result in sinus node dysfunction.3 Careful preoperative screening with 24-hour Holter monitors and electrocardiography (ECG) can help select the patients who would benefit from prophylactic epicardial lead placement.
Epicardial pacing, the predominant method of pediatric pacing until relatively recently, is now used mainly for patients in whom transvenous pacing is contraindicated or who are undergoing concomitant heart surgery. Some of the contraindications to transvenous pacing include prosthetic tricuspid valves, right-to-left intracardiac shunts, CHD or surgery precluding transvenous access to the cardiac chambers, and small patient size.4 Although there are no absolute technical limitations to a transvenous route except in premature infants, venous capacitance and lead failure resulting from growth are important considerations. Although each institution should make individual decisions on the basis of local procedural capabilities and experience, we generally consider transvenous pacemaker implants in children weighing more than 10 kg.
Epicardial leads are available with sutureless (screw-on) or suture-on methods of fixation. Alternatively, a standard transvenous lead can be used in postoperative CHD patients with epicardial scarring. The transvenous lead can be placed via a transmural technique, with the lead passed through the myocardial wall and attached to the endocardium.5 The steroid-eluting suture-on epicardial leads are our preferred lead, because the steroid can suppress the subacute threshold rise resulting from tissue inflammatory response. Several studies have shown that these steroid-eluting leads have good intermediate-term performance, with stable acute and chronic pacing and sensing thresholds and longevity similar to transvenous leads.6–9 However, screw-on leads may be preferable for patients who have undergone prior heart surgery, as the depth of scarring may hinder suture-on techniques.
The surgical access can be from a midline sternotomy, a left thoracotomy, or a subxiphoid approach, or via video assisted thoracoscopy.10 Each method has advantages, but the aim is to allow implantation of the proper number of leads in an individual patient. Finding an optimal site for maintenance of acceptable long-term pacing and sensing thresholds can be difficult because of bleeding, limited myocardial access, myocardial scarring, and pericardial adhesions. In patients with prior extensive right atrial (RA) surgery, left atrial pacing sites and Bachmann’s bundle in general have better chronic pacing and sensing thresholds than do right lateral or anterior atrial sites.11 A left thoracotomy can be used for implantation of left atrial epicardial leads in children with CHD.12 In small infants, short leads (15 to 25 cm) should be used because long leads left in the pericardial space can ensnare the heart, as noted in several case reports of cardiac strangulation or constriction of a great vessel by pacing leads.13,14
The site of pacing is being recognized as an important determinant of ventricular performance. The systemic ventricle is the chamber of choice to pace and best if it is performed in an apical location.15 Anular and high outflow tract locations produce worse dyssynchrony. The effects of pacing site on ventricular performance depend on baseline ventricular function. If ventricular dysfunction is present, it is better to expand the surgical entry access than to pace at a site known to produce ventricular dysfunction.
Once the leads are placed and tested, the pacemaker is typically set in a subrectus pocket, but in very small infants (<3 kg), the generator and leads can be left in the pleural cavity. Although uncommon, the abdominal-positioned generators can migrate into the pericardium,16 peritoneum,17 or pelvic space, a complication most often seen in very small infants.
Cardiac Resynchronization Therapy
Indications for CRT are well established for adults with normal cardiac anatomy, but not so for children and patients with CHD.18,19 The standard adult indication for CRT is a QRS duration of greater than 120 msec, an ejection fraction of less than 35%, and class II heart failure. Unfortunately, this combination rarely occurs in children. Although 90% of adults meeting these criteria have left bundle branch block, in patients with CHD such as tetralogy of Fallot, it is more common to have right bundle branch block and right ventricular (RV) dysfunction. The benefits of biventricular or dual-site pacing for these “right-sided” abnormalities must still be tested.
Types of CRT available are biventricular, dual-site, multisite, and temporary. In biventricular pacing, two distinct ventricles are present, with a pacing lead on each ventricle. If two sites on the same ventricle are paced, then it is called dual-site pacing. When the systemic ventricle is a single ventricle, then dyssynchrony can be decreased by pacing two widely separate sites on the same ventricle, a maneuver called multisided pacing. For children, temporary CRT has been used to improve cardiac output in the early postoperative setting.20,21
Implantable Cardiac Defibrillators
Indications for implantable cardiac defibrillators’ (ICD) implantation are listed in Box 132-2. The majority of ICDs are implanted via the transvenous route, but this may not be possible in small patients or those with anatomic constraints. Because ICD leads are larger and prone to fibrosis at the coils, patient size limitations for a transvenous system are different from those for a pacemaker. We try to limit transvenous ICD implantation to children who weigh more than 30 kg.
Box 132–2 Recommendations for ICD Therapy in Children, Adolescents, and Patients With Congenital Heart Disease
Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Source: American Heart Association, Inc.
Class I
Class III
Epicardial patches were once used regularly, but they were prone to breakage, infection, and pericardial restriction. Newer techniques use coils placed in subcutaneous or pericardial positions.22 The four coil types currently available are the subcutaneous coil, superior vena cava (SVC) coil, standard transvenous lead, and a three-finger subcutaneous array.
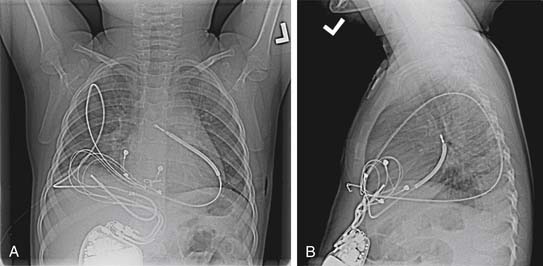
In the basic implantation sequence, a bipolar epicardial pacing lead is first placed, followed by a coil in the pericardial or pleural cavity. When a minimally invasive procedure is used, an active fixation transvenous lead is placed blindly into the posterior pericardial space, followed by extension of the screw to hold the coil in a stable position. The pace or sense connector is then capped. Intraoperative fluoroscopy or portable chest radiography is used to confirm proper lead position. If full exposure of the heart is available, a 5-cm SVC coil can be sutured directly in position. In levocardia, the best configuration is a high left lateral lead and right subrectus pocket (Fig. 132-1). Finally, defibrillation testing is performed, and if the defibrillation threshold is inadequate, then a second coil (length, 25 or 5 cm) is placed in a subcutaneous or pericardial location on the opposite side.
Cardiac Sympathetic Denervation
Left cardiac sympathetic denervation (LCSD), also known as left cervical stellate ganglionectomy, was first described in 1971.23 Although considered a highly effective method of surgical antiadrenergic therapy, its clinical use has been limited to a narrow set of indications. The procedure has been primarily used at a few centers for patients affected by the long QT syndrome (LQTS), and who are suboptimally controlled with standard medical therapy, usually consisting of β-adrenergic blocking medications. A recent multicenter report on a series of LQTS patients having undergone LCSD documented the efficacy of this intervention.24 It reported on 147 patients having undergone LCSD, with 99% having been symptomatic and 48% having suffered an aborted cardiac arrest before the operation. On a mean follow-up of 7.8 years after LCSD, 46% were asymptomatic and ICD shocks decreased by 95%.
A more recent and emerging indication is in patients with catecholaminergic polymorphic ventricular tachycardia (CPVT), a disorder of abnormal myocardial calcium homeostasis,25 characterized by life-threatening ventricular arrhythmias triggered during states of high sympathetic output. Patients with CPVT typically have structurally and functionally normal hearts and a normal baseline electrocardiogram, including a normal QTc measurement.
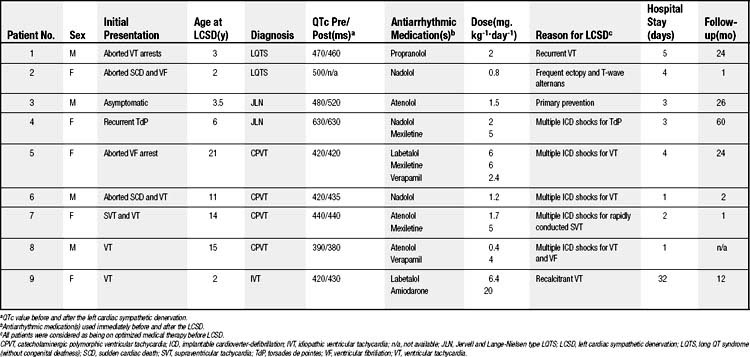
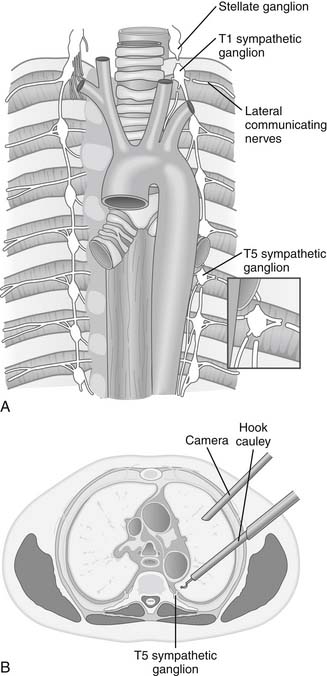
The surgical technique used in performing the LCSD varies among centers.24 Li and colleagues26 reported the first small series of LQTS patients undergoing LCSD using the video-assisted thoracoscopic surgery (VATS) approach. We recently reported our experience27 with nine young pediatric patients who underwent high left thoracic sympathetic denervation using VATS, of whom four were diagnosed with CPVT, four had LQTS, and one had idiopathic medically refractory ventricular tachycardia (VT) (Table 132-1). The LCSD procedure on all patients was performed by means of a left-sided VATS approach. After left lung isolation with a double-lumen endotracheal tube or the use of a bronchial blocker, the patient was positioned in a partial right lateral decubitus position. Three small stab incisions were made in the left side of the chest along the midaxillary line, with one incision at the level of the third intercostal space, one at the fourth, and one at the fifth. The first incision was used for the camera, the second for a grasper (or lung retractor if needed), and the third for the electrocautery hook dissector (Fig. 132-2B). After identifying the structures in the apex of the posterior chest wall and the heads of the ribs, the pleura was incised medial to the heads of the ribs, and the sympathetic chain was identified from the level of about T1 to T5 (see Fig. 132-2A). Using electrocautery, the sympathectomy involved transection of the left sympathetic chain at the level of T1, sparing the superior aspect of the stellate ganglion, and then at T5, as well as transection of the associated lateral nerves of Kuntz between those levels.
ARRHYTHMIA THERAPY IN ADULTS WITH CONGENITAL HEART DISEASE
The number of adult patients with CHD continues to increase. In 2001, it was estimated that more than 800,000 adults in the United States live with CHD.28 Many of these patients bear the weight of many decades of long-standing alterations in the volume- and pressure-loading conditions that result from their heterogeneous and often palliated anatomic arrangements. Approximately 50% are classified as having moderate or severe disease (e.g., tetralogy of Fallot, Ebstein’s anomaly, single-ventricle palliations). Although arrhythmias can develop with even mild disease, the incidence is highest for patients in the moderate and severe categories. As a result of the substantial and cumulative disease burden, cardiac arrhythmias late in life are a substantial source of morbidity, hospital admissions, and mortality for adults with CHD.29 They exhibit the full spectrum of arrhythmia issues, and their complex management presents challenges that are often considerable and shared equally by cardiologist and cardiac surgeon.
Electrophysiologic Substrates in Congenital Heart Disease
Intra-atrial Reentrant Tachycardia (Atrial Flutter)
The most common mechanism for symptomatic tachycardia in the adult CHD population is macroreentry in atrial muscle.30 The terms intra-atrial reentrant tachycardia (IART) and incisional tachycardia have become common labels for this arrhythmia so as to distinguish it from the typical variety of atrial flutter that occurs in structurally normal hearts.31–33 Generally, IART tends to be slower than typical flutter, with atrial rates in the range of 150 to 250 per minute. In the setting of a healthy AV node, such rates frequently conduct in a rapid 1:1 A-to-V pattern that can result in hypotension, syncope, or possibly cardiac arrest.34,35 Even if the ventricular response rate is safely titrated, sustained IART can cause debilitating symptoms in some patients from loss of AV synchrony, and it may contribute to thromboembolic complications36 when the duration is protracted. Usually, IART appears many years after operations that involved an atriotomy or other surgical manipulation of RA tissue. It can follow simple procedures such as closure of an atrial septal defect on occasion, but the incidence is higher among patients with advanced dilation, thickening, and scarring of the right atrium.37,38 Other risk factors for IART include concomitant sinus node dysfunction (tachy-brady syndrome) and older age at time of heart surgery.39
Thus, IART is a particular problem for older patients who have undergone the Mustard, Senning, or older-style Fontan operations, in which extensive suture lines and long-term hemodynamic stress result in markedly abnormal atrial myocardium. The route of propagation for an IART circuit varies according to the anatomic defect and type of surgical repair.40 It is usually restricted to RA tissue (regardless of the surgical destination of this tissue) and is modulated by regions of fibrosis from suture lines or patches, which function in combination with natural conduction barriers (crista terminalis, valve orifices, and the superior and inferior caval orifices) to channel the wave front along a macro-reentrant loop.41,42 If a tricuspid valve is present, the isthmus between the valve ring and the inferior vena cava is a common component of such circuits, but when the tricuspid valve is absent or otherwise deformed, the circuits follow less predictable paths that can be deciphered only by formal electrophysiologic mapping. Quite often, multiple IART circuits can be present in the same patient.43 Once recognized, IART can be reliably terminated with electrical cardioversion, overdrive pacing maneuvers,44 or administration of certain class I or III antiarrhythmic drugs.
The far more difficult task is prevention of recurrence. Multiple strategies have been developed for IART prevention, all of which can have value in selected patients, but none of which represents a universal solution. If the episodes are infrequent, well tolerated, and recognized promptly, it may be sufficient to rely on periodic cardioversion before embarking on more involved therapy. However, if IART episodes become frequent, cause significant symptoms, or are associated with atrial thrombus formation, aggressive treatment is indicated. The therapeutic options for IART include (1) antiarrhythmic drugs, (2) pacemaker implantation to correct bradycardia or provide automatic atrial antitachycardia pacing, (3) catheter ablation, and (4) surgical intervention with a modified atrial maze operation. The choice must be tailored to the hemodynamic and electrophysiologic status of the individual patient. Chronic antiarrhythmic drugs are still prescribed in some cases, but the broad experience with pharmacologic therapy for this condition has been discouraging34,45 even when potent agents such as amiodarone are used. Pacemaker implantation may be reasonable for patients with a picture of tachy-brady syndrome.35 Pacemakers with advanced programming features that incorporate atrial tachycardia detection and automatic burst pacing to interrupt reentry may also be beneficial in select cases.35,46 Catheter ablation is now used at many centers as an early intervention for IART.47 The technique has evolved rapidly since the introduction of three-dimensional mapping for improved circuit localization.31,48 When these technologies are combined with good anatomic definition and traditional electrophysiologic mapping maneuvers, short-term success rates of nearly 90% can be achieved.
Unfortunately, later tachycardia recurrence is still disappointingly common. The recurrence risk is particularly high (nearly 40%) among Fontan patients, who tend to have the largest number of IART circuits and the thickest or largest atrial dimensions. Although far from perfect, ablation outcomes for IART are likely to improve with continued experience and even now are far superior to the degree of control obtained with medications alone. Furthermore, even if IART episodes are not eliminated entirely by ablation, the procedure can often provide substantial improvement by reducing the frequency of episodes and eliminating the need for ongoing drug therapy.48 If these measures fail to prevent IART, or if the patient is returning to the operating room for hemodynamic reasons, consideration should be given to surgical ablation during an RA maze operation. This procedure is used most often for the Fontan population with the most refractory variety of IART and is usually combined with revision of the Fontan connection from an older atriopulmonary anastomosis to a modern cavopulmonary connection in the same setting.49
The Fontan operation for patients with single ventricle results in extensive suture lines and abnormal hemodynamics that predispose patients to atrial tachycardias and sinus node dysfunction. Up to 50% of patients with single ventricle who have undergone older-style Fontan operations develop atrial tachycardia within a decade of surgery. Fontan conversion has been proposed for patients suffering from a variety of late sequelae, including conduit and vascular obstruction, atrial arrhythmias, protein-losing enteropathy, and thrombotic complications. However, documented benefits have been limited to a small number of centers and over short follow-up periods, compared with the relatively extensive data available on initial Fontan procedures.50,51 Fontan conversion can provide symptomatic improvement in patients with late complications associated with this circulation, including atrial tachycardias.52–54 Criteria for optimal patient selection and anticipated clinical outcomes remain controversial. Conversion alone does not appear to protect against recurrent atrial tachycardias,55–57 whereas concomitant maze surgery may suppress postoperative tachycardia.58–60 Several different surgical approaches have been reported in this heterogeneous patient population. The most recent series from eight centers that have reported outcomes on 10 or more patients have noted variable conversion failure rates, with an aggregate rate of death or transplantation of 8.4% in follow-up of a total of 203 patients.53,54,61–67 Significant long-term morbidities, such as renal failure, thrombotic events, and arrhythmia, have likewise been reported.
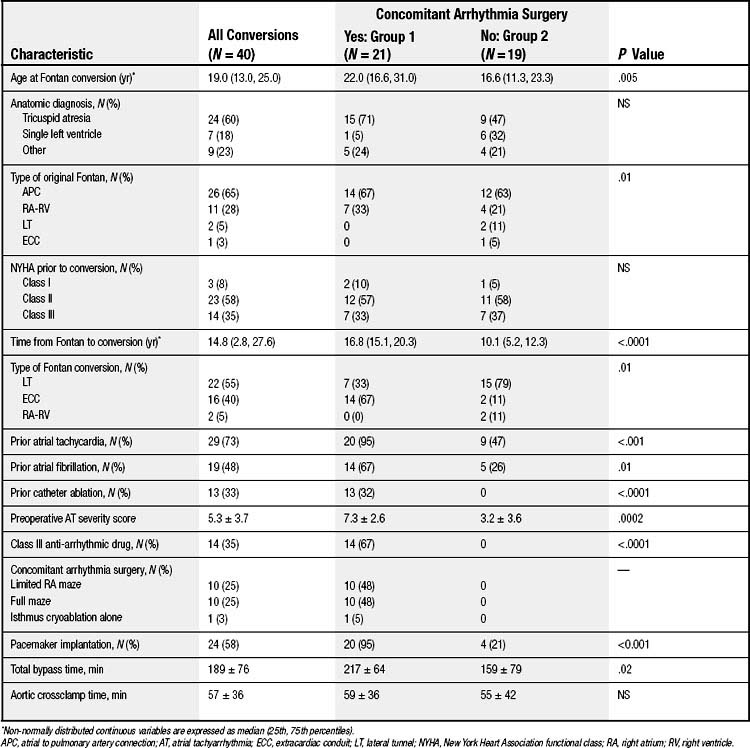
At Children’s Hospital Boston, 40 patients underwent Fontan conversion between 1990 and 2006, 21(53%) with and 19 (48%) without concomitant arrhythmia surgery.68 Table 132-1 summarizes baseline characteristics and surgical considerations in all patients and according to whether or not they had arrhythmia surgery. Clinical indications for conversion included surgically correctable anatomic lesions (n = 28), thrombi (n = 4), intracardiac right-to-left shunting (n = 4), and medically refractory atrial tachyarrhythmia (n = 29), with 16 patients having more than one indication. Four patients had protein-losing enteropathy. In the 39 patients with preoperative catheterization, the ventricular end-diastolic pressure was 9.8 (1.0, 22.0) mm Hg, with four having values greater than 12 mm Hg. Preexisting atrial arrhythmias were present in 29 (73%) patients; 10 had macro-reentrant atrial tachyarrhythmia, and 19 had atrial fibrillation (paroxysmal in 12; persistent in 7). Surgical characteristics are summarized in Table 132-2. Twenty-two patients (55%) had a 4-mm fenestration at the time of conversion. Resection of a large portion of the anterior RA wall was performed on 20 patients (50%). All patients with a biatrial maze procedure had a preoperative history of atrial fibrillation. Of 19 patients with a history of AF, 10 underwent a full maze and four had a limited RA maze procedure. Eighteen patients (45%) had concomitant pacemaker implantation (including six with previously implanted devices), and six additional patients required pacemaker placement postoperatively. Perioperative complications, including bleeding, thrombosis, seizures, and renal dysfunction requiring peritoneal dialysis, were observed (Table 132-3).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree