CHAPTER 53 Applications of Cardiovascular Magnetic Resonance and Computed Tomography in Cardiovascular Diagnosis
Cardiovascular magnetic resonance (CMR) and cardiovascular computed tomography (CCT) are increasingly used in the diagnosis and management of cardiovascular disease.1–3 Both of these imaging modalities have overcome similar challenges posed by cardiac and respiratory motion and the demand for high temporal and spatial resolution to enable noninvasive imaging that aids in the diagnosis and management of a variety of cardiovascular disorders. In addition, CMR and CCT have unique capabilities that permit great flexibility, precision, and reproducibility in the acquisition and display of anatomic and functional data that are useful for surgical diagnosis, planning, and follow-up.
IMAGING COMPARISONS
The advantages and limitations of CMR and CCT complement those of other imaging techniques, such as echocardiography, x-ray angiography, and radionuclide imaging. Compared with echocardiography and radionuclide imaging, CMR and CCT offer superior anatomic scope and spatial resolution. CMR is the reference standard for evaluation of left ventricular cavity size, systolic function, and mass, providing highly reproducible measures and enhancing noninvasive follow-up of disease processes.4 CCT measures of left ventricular cavity size and systolic function compare favorably with CMR.5 In contrast to echocardiography and nuclear imaging, CMR permits unrestricted image acquisition orientation, which can be readily adjusted to particular patient and study requirements. CCT acquisitions are always in the axial plane. Isotropic resolution facilitates post-processing reconstruction in any desired orientation. There is also much advanced post-processing software for CCT. In contrast, echocardiography offers the advantages of portability, lower cost, lack of ionizing radiation, widespread availability, greater ease of patient monitoring, and greater sensitivity for structures with chaotic motion, such as vegetations. Whereas CMR and CCT myocardial perfusion techniques have been shown to provide useful qualitative and quantitative data, they have not yet been clinically validated to provide the diagnostic and prognostic information proven for radionuclide techniques. Further comparisons between CMR, CCT, and other techniques will be made in the following sections dealing with specific types of examinations.
IMAGING PRECAUTIONS
Precautions generally applicable to magnetic resonance imaging are applicable to CMR. Before imaging, all patients must undergo detailed screening for any potential contraindications to magnetic resonance scanning. In addition to general concerns of metallic implants and severe claustrophobia, patients should be screened for the presence of any incompatible material. Excluded devices include some that are relatively common among those with cardiovascular disease, such as pacemakers, retained permanent pacemaker leads, and implantable cardioverters-defibrillators. Bioprosthetic and mechanical heart valves, sternotomy wires, thoracic vascular clips, and intracoronary stents are generally considered CMR safe at field strengths up to 3 T (see www.mrisafety.com), although they may produce local artifacts that reduce image quality.6
Because of bulk cardiac motion during systole and diastole, most CMR protocols require ECG triggering with images composed from data collected during multiple successive cardiac cycles. Despite this, good functional image quality can frequently be obtained among patients with atrial fibrillation,7 although image quality may be impaired among subjects with frequent and irregular premature beats. Among patients with irregular rhythms, non–ECG-gated real-time imaging CMR (which permits real-time image acquisition analogous to two-dimensional echocardiography but at lower spatial and temporal resolutions than those attained with a gated CMR technique) can provide useful information.8 CCT data are generally of poor quality in the setting of atrial fibrillation and frequent ectopy,9,10 although acquisitions with 256- or 320-slice machines over a single heart beat may provide better image quality.
As noted earlier, CMR generally does not require the use of an exogenous contrast agent. When needed, however, gadolinium-containing CMR contrast agents have a much more favorable safety profile in regard to both nephrotoxicity and anaphylaxis compared with iodinated agents used in x-ray angiography and computed tomography.11 Administration of gadolinium contrast to patients with severe renal dysfunction may result in nephrogenic systemic fibrosis, a very rare but severe disorder that may result in death.12,13 Patients are typically screened with a questionnaire to identify those who may potentially have renal disease and who require a determination of the estimated glomerular filtration rate. Patients with mild renal impairment (estimated glomerular filtration rate of 30 to 60 mL/kg/1.73 m2) can be imaged with a reduced dose of contrast agent. Alternative imaging modalities are usually used in those patients with moderate to severe renal dysfunction (estimated glomerular filtration rate of less than 30 mL/kg/1.73 m2), especially in the setting of dialysis therapy.
Because of the general requirement for iodinated contrast agents, CCT must be performed with caution in patients with diabetes or mild renal insufficiency. Alternative noninvasive imaging methods are preferable in the setting of moderate to severe renal insufficiency unless dialysis has already been instituted. The use of saline, bicarbonate solution, and N-acetylcysteine may reduce the incidence of acute renal failure due to iodinated contrast material14–16 but may not be effective in patients with severe renal dysfunction.17,18
Radiation exposure is a significant consideration in the use of CCT. Typical helical acquisitions with a 64-slice machine are associated with an effective dose of 15 to 21 mSv, which is associated with a nontrivial risk of cancer that is higher in women and in younger patients.19 Dose modulation (reducing the radiation dose during ventricular systole) reduces the effective dose to 5 to 10 mSv.19 Imaging with lower energy radiation reduces the dose an additional 15% to 20%.20 Prospective gating with use of a step-and-shoot mode of imaging is associated with a relatively low radiation exposure of 4 to 6 mSv, but it does not perform imaging over the entire cardiac cycle.21 Increasing awareness of the radiation exposure associated with CCT has led to the adoption of one or more of these techniques at most centers. For middle-aged and younger patients with chronic disorders, potential radiation exposure for the expected multiple tests over their lifetimes should be considered.
CLINICAL APPLICATIONS
Diseases of the Thoracic Aorta
CMR and CCT are widely used clinically for the assessment of thoracic aortic aneurysm and dissection. With CMR, the structure of the aorta is delineated by a combination of the following protocol components in the transverse, coronal, sagittal, and oblique planes: (1) ECG-gated spin echo imaging, which reveals the aortic wall with rapidly flowing blood appearing black and thrombus and slowly moving blood appearing gray; (2) ECG-gated steady-state free precession (SSFP) imaging, which produces bright blood images in single-shot as well as in cine acquisitions; and (3) three-dimensional (3D) CE-MRA with a gradient echo acquisition. Temporally resolved CE-MRA is particularly useful to minimize motion artifacts that would otherwise cause nondiagnostic or false-positive results. With CCT, imaging of the aorta involves a larger image acquisition volume to include the entire thoracic aorta and can be performed with or without ECG gating. Gating is preferred for the evaluation of dissection to avoid motion artifacts that can mimic a dissection flap.21
Aortic Aneurysm
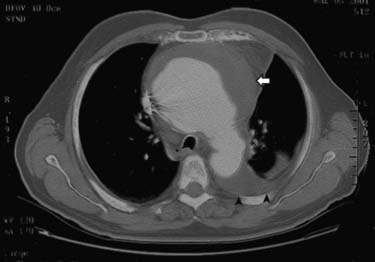
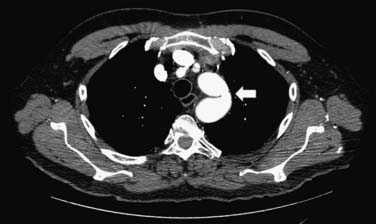
CMR and CCT are also superior methods for identification of true and false thoracic aortic aneurysms. In true aneurysms, the aneurysmal aortic wall is composed of intima, media, and adventitia. False aneurysms represent a contained rupture of the intima and media, with only the adventitia and periadventitial connective tissue limiting the hemorrhage (Fig. 53-1). False aneurysms generally have a narrow “neck” or communication with the main aortic lumen. True aneurysms are more commonly fusiform (bulge aligned along the long axis of the aorta; Fig. 53-2) than saccular (sacklike bulge extending from a side of the aortic wall). CE-MRA reveals the presence and extent of these lesions as well as any associated thrombus. CCT is recommended as the imaging modality of choice for most patients in the assessment of acute disease; 3D CE-MRA is recommended for most patients with chronic disease.22 As with aortic dissection, advantages of CMR and CCT assessment compared with x-ray angiography include the capability to evaluate for associated complications, such as hemopericardium and left ventricular dysfunction. CMR can also assess for the presence of associated aortic regurgitation. After composite graft replacement of the ascending aorta, CMR is useful for detection of postoperative complications, such as leakage or hematoma formation.23,24
Aortic Dissection
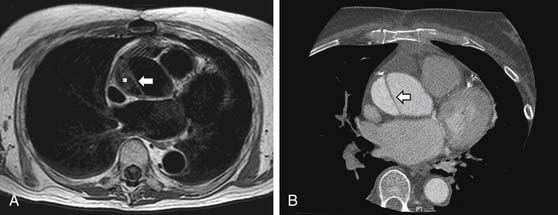
CMR and CCT, along with transesophageal echocardiography (TEE), are the primary methods used to diagnose and to monitor patients with acute or chronic aortic dissection. Because each of these imaging modalities has high diagnostic accuracy for dissection, the selection among these methods is generally governed by the condition of the patient, institutional access, and local expertise. Both CMR and CCT offer a good combination of sensitivity and specificity (sensitivity above 95% and specificity above 90%) for dissection25 and provide information about involvement of major branch vessels and all segments of the aorta, unlike TEE, which is limited to the thoracic aorta and by the adequacy of acoustic windows (particularly for the segment of ascending aorta anterior to the trachea). All three methods provide useful information about pericardial involvement. CMR can also assess aortic valve integrity, as can TEE. The main disadvantages of CMR in the acute setting are potential obstacles to continuous monitoring and care of an unstable patient during transport and performance of the study and the requirement that the patient remain motionless during the examination. CCT is frequently available in the emergency department and is therefore the most common initial imaging modality chosen to diagnose acute aortic dissection.26 However, CMR is considered the imaging procedure of choice27 for serial follow-up of the medically or surgically treated patient with dissection according to the recommendations of the task force on aortic dissection of the European Society of Cardiology (which have been endorsed by the American College of Cardiology) because of the lack of radiation exposure.28 Follow-up is recommended after hospital discharge at 1 month, 3 months, 6 months, 12 months, and yearly thereafter.28 Accurate interpretation of postoperative images requires knowledge of the surgical procedure performed and the expected range of routine postoperative sequelae, including thickening around the graft and presence of thrombus outside the graft and within the native aortic wrap.29
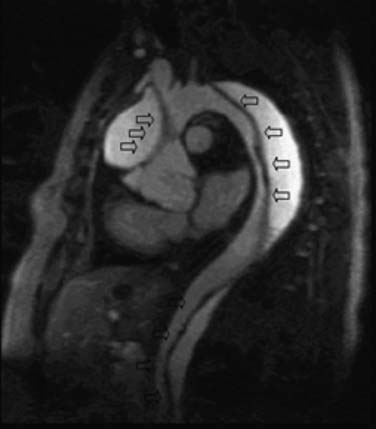
CCT aortic assessment is typically completed in less than 1 minute and often in less than 30 seconds; CMR aortic assessment can often be completed within 20 minutes. Both can display the location and extent of dissection identified as an intimal flap separating true and false aortic lumens along with sites of intraluminal communication and can readily assess involvement of the aortic root, arch vessels, and renal arteries (see Fig. 53-2). CMR spin echo images may identify relatively bright regions within the true or false lumen attributable to stagnant blood flow or thrombus. Gradient echo images demonstrate flap motion and blood flow in the true and false lumens. 3D CE-MRA is highly sensitive for dissection30 and can be implemented with subsecond temporal resolution to obviate the need for a breath hold (Fig. 53-3).31 Alternatively, SSFP imaging without administration of an exogenous contrast agent can be accomplished within 4 minutes and may suffice (Fig. 53-4).32 Left ventricular function and involvement of the proximal coronary arteries can be assessed by CCT with a gated acquisition or CMR with additional cine and coronary imaging. CMR can also assess the presence of aortic regurgitation (by cine imaging of the left ventricular outflow tract) and the size of the regurgitant fraction (by a phase velocity encoding acquisition at the base of the aortic root).
Aortic Intramural Hematoma
Intramural hematoma can be identified by a localized thickening, frequently crescentic or circular, within the wall of the aorta, interposed between intima and media, with characteristics of an acute or subacute collection of blood.33 Whereas both CMR and CCT can identify intramural hematoma,34 CMR has been found to be superior to CCT in distinguishing acute intramural hematoma from atherosclerotic plaque and chronic intraluminal thrombus.35 Acute hemorrhage appears isointense or more intense compared with the aortic wall on T1-weighted images and displays high signal intensity on T2-weighted images.33,36 In contrast, subacute hemorrhage displays high signal intensity on T1 images and less signal intensity on T2 images. The layer of displaced calcified intima overlying the hematoma generally produces a relatively smooth surface concave to the lumen (crescent shape), which may help distinguish this entity from protuberant, frequently irregularly shaped atherosclerotic plaque.
Sinus of Valsalva Aneurysm
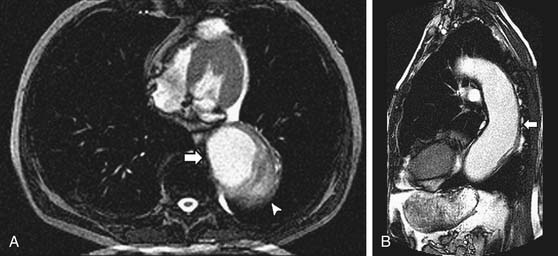
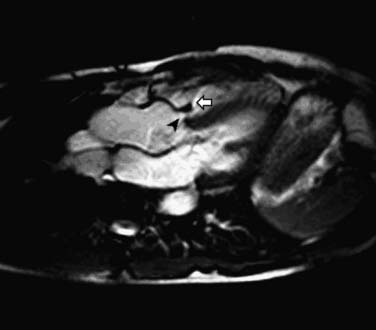
A sinus of Valsalva aneurysm is often first suspected on transthoracic echocardiography and can be readily visualized by CCT, CMR, and TEE as an enlargement or outpouching (frequently creating a “windsock” appearance) of a sinus of the aortic root. CMR and echocardiography offer the advantage of providing information about blood flow (e.g., rupture of the aneurysmal sinus into an adjacent chamber) in addition to anatomy (Fig. 53-5). Shunt flow through the rupture is generally present both in diastole and in systole. The magnitude of the shunt may be calculated by flow velocity encoding data.
Atherosclerotic Plaque and Aortic-Penetrating Ulcer
CMR, CCT, and TEE can provide qualitative and quantitative information about the presence, thickness, and distribution of atherosclerotic plaque in the aorta.37–39 Complex plaque is generally defined as protuberant plaque at least 4 mm in thickness or plaque with mobile elements because plaque with these characteristics is associated with embolic risk.40 Plaque thickness can readily be ascertained by CMR or CCT, although overlying mobile elements are best assessed by TEE because of their chaotic motion and relatively small size.41 Ascending aortic plaque is a predictor of adverse cerebral outcomes after coronary artery bypass grafting. Preoperative (CMR or CCT) or intraoperative (TEE) identification of such plaque may prompt alteration in surgical strategy or technique.40,42
Aortic ulceration occurs in regions of atherosclerotic plaque. Penetrating ulcers are described as those breaching the internal elastic lamina with associated hematoma formation in the media.43,44 CMR and CCT images visualize the position and shape of such ulcers and accompanying adjacent intramural hematoma.35,39,41 Penetrating ulcers are also frequently associated with aortic aneurysm formation.44,45 Associated chest or back pain is a significant risk factor for progression to pseudoaneurysm or free rupture.44
Takayasu’s Arteritis
Takayasu’s arteritis is a chronic idiopathic vasculitis that primarily affects the aorta and its branches. It is most prevalent among Asian women younger than 40 years.46,47 The aortic arch (or distal aorta) and its branches as well as the pulmonary arteries have characteristically tapered narrowings or occlusions with areas of dilatation. The availability of noninvasive imaging is important among these patients, who require long-term follow-up to guide medical and surgical therapy. These lesions have been traditionally detected by conventional x-ray angiography, but CMR and CCT can accurately display these lesions and also provide information about a vessel wall abnormality.48,49 CMR is generally preferred because of the need for repeated imaging in these younger subjects. Evidence of vessel wall edema is common but does not correlate well with subsequent lesion development.50 Therefore, the current role of imaging in this disease is to identify the characteristic angiographic lesions.49
Congenital Aortic Anomalies
Aortic coarctation is characterized by a ridge of medial thickening and intimal hyperplasia along the posterolateral aortic wall. Coarctation most commonly presents just distal to the left subclavian, occurring more rarely just proximal to the left subclavian. Anatomic assessment with CMR or CCT reveals the location of the coarctation and associated collaterals, usually assessed in an oblique sagittal view aligned with the descending and ascending thoracic aorta (Fig. 53-6).51 Typical CMR protocols use spin echo, gradient echo, or CE-MRA techniques.52,53 Additional cardiac lesions that frequently accompany coarctation can also be identified, including bicuspid aortic valve (imaged in cross section) and ventricular septal defect (imaged in a horizontal long-axis or four-chamber view). Imaging is also useful for follow-up after surgical repair or balloon angioplasty,52,54 and routine CMR follow-up has been recommended.55 Potential complications that may be visualized include renarrowing and aneurysm or pseudoaneurysm at the repair site.
Whereas patent ductus arteriosus can usually be identified by transthoracic echocardiography, CMR or CCT may be useful when echocardiographic images are nondiagnostic due to poor acoustic windows.56,57 For both patent ductus arteriosus and atrial or ventricular septal defects, CMR can provide an assessment of the pulmonic-to-systemic flow ratio (Qp/Qs) by applying the flow velocity encoding technique.58
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree