Chapter 110
Aortoiliac Disease
Direct Reconstruction
Matthew T. Menard, Michael Belkin
Based on a chapter in the seventh edition by Matthew T. Menard and Michael Belkins
The clinical manifestation of atherosclerosis involving the abdominal aorta and iliac arteries is one of the most common therapeutic challenges encountered by vascular surgeons. British anatomist and surgeon John Hunter first appreciated the implications of arterial occlusive disease of the aortic bifurcation in the late 1700s. His dissection specimens remain on view at the Hunterian Museum in London and laid the groundwork for Leriche’s later appreciation of the disease process that bears his name.1
The modern era of surgical reconstruction for complex atherosclerotic occlusive disease began in 1947 with the successful endarterectomy of a heavily diseased common femoral artery (CFA) by Portuguese surgeon dos Santos.2 Wylie et al3 in San Francisco extended this new technique to the aortoiliac (AI) level 4 years later, but it would be another 10 years before synthetic grafts were regularly used for aortic bypass grafting. Early preference for aorta–to–iliac artery grafting would eventually be supplanted by the more durable aortobifemoral bypass (ABFB) procedure.
In recent years, tremendous advances in our understanding of the biology of atherosclerosis and in our ability to treat arterial occlusive disease percutaneously have had a dramatic impact on the treatment algorithms for peripheral arterial disease as a whole and for AI disease in particular. The growing range of treatment options allows us to tailor therapy to the particular clinical situation, and ongoing improvements in graft material, surgical technique, and perioperative management have contributed to a steady decline in postoperative morbidity and mortality. Given the advent of less invasive therapeutic options, traditional aortobifemoral grafting is increasingly being performed for more complex patterns of disease or as a secondary or tertiary procedure in the setting of recurrent disease.4 High levels of patient satisfaction and excellent long-term outcomes, however, remain the hallmark of aortobifemoral surgical revascularization. This chapter reviews the role of direct operative reconstruction in the current surgical management of AI arterial occlusive disease.
Pathology and Clinical Presentation
Pathology
AI disease typically begins at the aortic terminus and common iliac artery origins and slowly progresses proximally and distally over time.5 Progression is variable but may ultimately result in total aortic occlusion (Fig. 110-1). In the setting of adequate collateralization, patients can tolerate their levels of claudication and can be successfully managed nonoperatively for many years. Approximately one third of patients operated on for symptomatic AI occlusive disease have significant orificial profunda femoris occlusive disease, and more than 40% have significant superficial femoral artery (SFA) disease. Disease can also extend to the level of the renal arteries (see Fig. 110-1). Although early reports indicated that up to one third of patients with aortic occlusion went on to develop renal artery thrombosis during a period of 5 to 10 years,6 later prospective studies failed to demonstrate a similar degree of compromise of the renal vessels in this population.7 Involvement of the renal or mesenteric vessels to a degree warranting concurrent repair is seen in only a minor proportion of patients with AI disease.
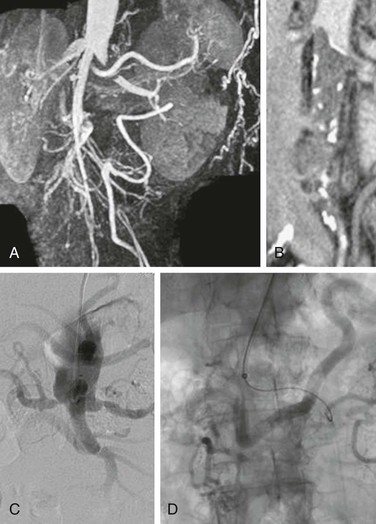
Figure 110-1 Two patients with total aortic occlusion extending to the level of the renal arteries. A and B, Coronal CT images in one patient demonstrate propagation of thrombus into the renal arteries (arrow). C and D, The second patient also has associated renal artery occlusive disease. Meandering mesenteric arteries are demonstrated in both patients.
Collateral Circulation
Although atherosclerotic disease limited to the AI region commonly gives rise to claudication of varying degrees, it is rarely associated with critical limb ischemia (CLI). This is largely the result of abundant collateralization around the point of obstruction, which reconstitutes the infrainguinal system with sufficient flow to ensure adequate resting tissue perfusion (Fig. 110-2). The primary compensatory networks develop from the lumbar and hypogastric feeding vessels and connect to circumflex iliac, hypogastric, femoral, and profunda recipients. Additional collaterals that arise in more extreme degrees of obstruction include the internal mammary artery–to–inferior epigastric connection and the superior mesenteric artery–to–inferior mesenteric artery and hemorrhoidal artery pathway. The latter connection comprises the arc of Riolan and the meandering mesenteric artery (Fig. 110-3); it is important to recognize the presence of such a large and significant collateral because it should be preserved during surgical reconstruction.
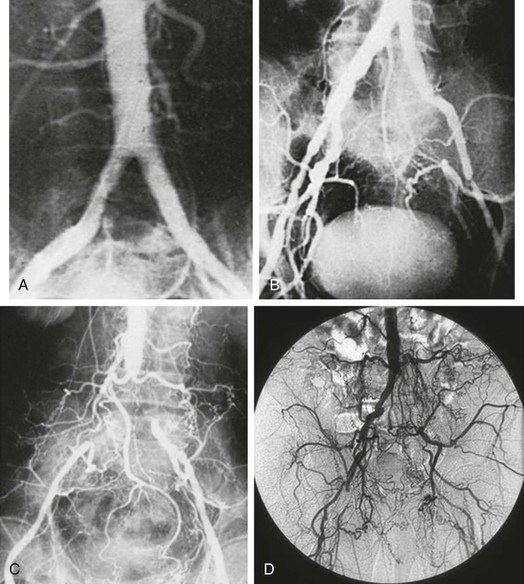
Figure 110-2 A–D, Aortoiliac occlusive disease of increasing severity, with a progressively well-developed pattern of iliolumbar to hypogastric and femoral circumflex collateralization. Male patients with a disease distribution illustrated in B–D may be impotent secondary to compromised hypogastric perfusion.
Embolism
Two well-recognized exceptions to the general observation that isolated AI occlusive disease does not result in CLI arise in the context of embolic disease. Large thromboemboli that arise from a cardiac or other proximal source and lodge at the aortic bifurcation, referred to as saddle emboli, can lead to profound acute bilateral lower extremity ischemia. The so-called blue toe syndrome, in contrast, occurs when atherosclerotic debris breaks free from an aortic or iliac plaque and embolizes to the distal vessels.8,9 Wire manipulation during coronary or peripheral angiographic procedures or surgical cross-clamping across a calcific aortic plaque can trigger such emboli. The terminal targets of the microembolic particles, composed of cholesterol crystals, calcified plaque, thrombus, or platelet aggregates, are typically the small vessels of the toes or heel. The syndrome is typically characterized by palpable pedal pulses in the presence of patchy ischemia (livedo), but more severe ischemia or gangrene of the proximal or distal forefoot may occur.
Epidemiology
The majority of patients presenting with atherosclerotic occlusive disease of the aorta and iliac vessels have diffuse disease involving multiple levels of the peripheral vasculature tree; in most cases, AI occlusive disease is found in combination with femoropopliteal or infrageniculate occlusive disease. Patients with isolated AI disease are generally younger and have a higher relative incidence of smoking and hypercholesterolemia as associated vascular risk factors.10 They are nearly as likely to be female as male and typically have a normal life expectancy.11 In contrast to this pattern, patients with more progressive multilevel disease are commonly older, more frequently have diabetes and hypertension, and are more likely to be male and to have concomitant cerebrovascular, coronary, and visceral atherosclerosis.10 Not surprisingly, patients with diffuse, multisegment disease often present with ischemic rest pain or more severe perfusion impairment, leading to tissue loss or gangrene as opposed to isolated claudication.12 Such patients manifest a significant reduction in life expectancy compared with their age-matched counterparts.11
A particularly virulent form of atherosclerotic arterial disease is often found in young women who smoke.13,14 Radiographic imaging in this subset of patients typically reveals atretic, narrowed vasculature with diffusely calcific atherosclerotic changes. Frequently, a focal stenosis is found posteriorly at or proximal to the aortic bifurcation (Fig. 110-4). In the setting of this particular disease distribution and characteristic patient profile, the term small aortic syndrome or hypoplastic aortic syndrome is used.15 Such patients invariably have an extensive smoking history but may be without other typical risk factors for atherosclerosis. The diminutive size of the aorta and iliac vessels has important treatment implications; the durability of either endovascular intervention or local endarterectomy is generally inferior in such patients, particularly in the presence of continued cigarette use.
Presenting Symptoms
Chronic obliterative atherosclerosis of the distal aorta and iliac arteries commonly is manifested as symptomatic arterial insufficiency of the lower extremities, producing a range of symptoms from mild claudication to more severe levels of CLI. Disease limited to the AI segment is seen in a minority of cases; more often, it is present in combination with occlusive disease of the femoropopliteal arteries or other arterial beds. Patients with hemodynamic impairment limited to the AI system may have intermittent claudication of the calf muscles alone or involvement of the thigh, hip, or buttock; patients with CLI usually have multilevel disease of the AI and infrainguinal arteries. Those presenting with claudication secondary to AI disease are, on average, nearly a decade younger than those with claudication stemming from infrainguinal occlusive disease.13 Up to 30% of male patients may have difficulty achieving and maintaining an erection owing to inadequate perfusion of the internal pudendal arteries.16 In men, the well-characterized constellation of symptoms and signs known as Leriche’s syndrome, associated with terminal aortic occlusion, includes claudication (thigh, hip, or buttock), atrophy of the leg muscles, impotence, and reduced femoral pulses.17 The equivalent impact of impaired pelvic perfusion in women remains poorly understood but has attracted investigative attention.18
Diagnosis
History and Physical Examination
The diagnosis of AI occlusive disease can usually be made after a careful history and physical examination (see Chapter 108). For a patient with multiple vascular risk factors, claudication (hip, buttock, or thigh), and absent femoral pulses, the diagnosis of AI disease is straightforward. In other patients, symptoms of claudication can sometimes be difficult to distinguish from those of hip arthritis or nerve root irritation stemming from lumbar disk disease or spinal stenosis.19
The variability of presenting signs and symptoms in patients with AI disease sometimes leads to diagnostic confusion. Although proximal claudication is most common, patients with AI occlusive disease in isolation or those with combined infrainguinal disease may present exclusively with calf claudication. Although the involved muscle groups may be atrophic from disuse in patients with claudication, the lower extremities frequently appear well perfused at rest. Palpable femoral and even pedal pulses may be detectable at rest, reflecting the presence of robust collateral networks; pulses may become diminished or absent only after a trial of exercise. Similarly, a palpable thrill or audible bruit may initially be absent at the lower abdominal or groin level but become detectable after exertion. Conversely, femoral bruits or diminished femoral pulses arising from CFA or profunda femoris stenotic disease can mistakenly be attributed to AI inflow disease.
Noninvasive Hemodynamic Assessment
Noninvasive arterial testing in the form of segmental systolic blood pressure measurements and pulse volume recordings can be helpful in confirming a clinical diagnosis of peripheral arterial disease and further defining the level and extent of obstruction (see Chapters 15 and 108).20 A difference of at least 20 mm Hg between the brachial pressure and the proximal thigh pressure reflects a significant stenosis in the aorta or iliac arteries, but it may be confused by proximal SFA occlusion. A further reduction in pressure between the thigh and ankle level is consistent with concomitant SFA, popliteal, or tibial outflow disease. Because patients with disabling symptoms occasionally demonstrate normal or near-normal ankle-brachial indices, repeating the pressure measurements after provocative graded exercise treadmill testing can be particularly useful if clinical suspicion of AI disease persists. Combined with a careful history and physical examination, noninvasive hemodynamic arterial studies usually provide sufficient data to select patients for revascularization. Further imaging is warranted only after the diagnosis of AI disease has been established and the clinical decision to intervene has been reached.
Imaging
Duplex Ultrasound
Duplex assessment of the AI, renal, and visceral arteries is labor-intensive and time-consuming and requires an institution with dedicated protocols and vascular laboratory personnel. Nevertheless, a number of institutions routinely obtain information of sufficient quality to be useful in preprocedure planning.21 In the absence of overlying bowel gas, obesity, or vessel tortuosity, the precise anatomic location of stenoses can be determined and their severity quantified. Indirect techniques to assess the resistive index are of some value in overcoming the commonly encountered limitations in renal arterial analysis and can be of utility in deciding whether a given lesion warrants concomitant revascularization at the time of aortic reconstruction.22 Duplex ultrasound (DUS) is particularly beneficial in patients with renal insufficiency, in whom the avoidance of contrast agents is advantageous. As the technology continues to be refined, as operator skill and training improve, and as novel adjunctive duplex imaging agents evolve, this modality is likely to play an increasing role in the management of patients with visceral and AI disease.23 At present, however, it remains inferior to other imaging techniques for preoperative planning and has not gained wide acceptance.
Axial Imaging
With new acquisition techniques, shorter acquisition times, and high-quality three-dimensional postprocessing capabilities, magnetic resonance angiography (MRA) has become particularly well suited to evaluation of the aorta and renal and mesenteric vessels. Indeed, several series have reported 100% sensitivity and specificity in detecting origin stenoses of the visceral vessels.24,25 In many centers, MRA has supplanted contrast angiography as the initial imaging study of choice (see Chapters 23 and 108). Advances have solved many of the technical limitations of earlier studies, and reliable roadmaps to guide percutaneous or operative planning are now obtainable and reproducible (Fig. 110-5). MRA continues to occasionally overestimate the degree of renal artery stenosis, albeit much less so than previously.
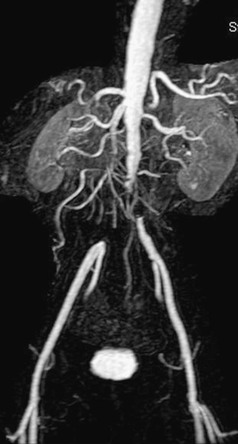
Figure 110-5 Total aortic occlusion demonstrated by MRA of sufficient quality to support surgical planning and to obviate the need for formal angiography.
In some institutions, computed tomographic angiography (CTA) is favored over MRA as a more clinically useful noninvasive alternative to standard angiography (see Chapter 22). Current-generation multislice technology has overcome many of the earlier limitations of computed tomography (CT),26,27 and images of exceptional clarity and submillimeter spatial resolution are now routinely available for the aortic and iliac arterial segments (Fig. 110-6). The recent development of 320-slice scanners, and those on the horizon that combine angiography and CT technology, is likely to push this modality closer to the forefront in the near future.
Arteriography
When a lesion in the aorta or iliac arteries amenable to percutaneous therapy is identified on MRA, CT, or duplex imaging, arteriography is then pursued. If good-quality imaging is obtained with MRA or CTA and the clinical situation or anatomic pattern is unfavorable to a percutaneous approach, AI reconstruction can frequently be planned directly from the information obtained by MRA or CTA, obviating the need for traditional subtraction angiography.28,29 Although DUS, CTA, and MRA have had a significant impact on preoperative planning algorithms for patients with AI disease, invasive contrast angiography remains the “gold standard” imaging modality if anatomic questions remain. Technical advances in catheters, contrast agents, radiographic equipment, and image processing have led to greatly improved safety and image quality (see Chapter 19).
In cases in which the decision has been made to proceed with surgical revascularization, angiography may be undertaken to obtain a final detailed roadmap of the relevant anatomy. Although a transbrachial approach is sometimes required,30 more often than not a standard retrograde femoral approach is possible, despite long-segment near-occlusive or occlusive aortic or biiliac disease. Supplemental lateral and oblique views of the abdominal aorta are advised to delineate possible concomitant mesenteric or renal artery occlusive disease. Specific attention should be directed to the inferior mesenteric artery; a large patent inferior mesenteric artery, particularly in the presence of superior mesenteric artery or hypogastric artery occlusive disease, may require preservation during aortic reconstruction to avoid potentially disastrous bowel ischemia. Multiple projections of the iliac and femoral bifurcations are essential to clarify the extent of disease in these regions because involvement of the hypogastric and external iliac arteries has significant implications in choosing the operative approach. Full runoff views of the lower extremities are also needed to assess the presence or absence of femoropopliteal or crural disease. In ambiguous cases, pullback pressure measurements, both before and after the administration of a systemic vasodilator such as papaverine or nitroglycerin or the application of a tourniquet to induce reactive hyperemia, can be useful in documenting the hemodynamic significance of a particular stenotic segment.31 A resting systolic gradient of approximately 5 to 10 mm Hg or a change in the systolic pressure greater than 15% after pharmacologic vasodilatation is a reliable indicator of disease warranting inflow revascularization.32
Indications for Surgical Intervention
Impact of Endovascular Treatment
A significant paradigm shift has occurred in the treatment of atherosclerotic arterial disease.19,33 Angioplasty and stenting have become first-line therapy for most patients with AI, renal, subclavian, and coronary occlusive disease.34 Whereas percutaneous treatment of the aorta and iliac arteries was previously limited to short-segment, TransAtlantic Inter-Society Consensus (TASC) type A or B iliac lesions, wire-based technology has now been successfully applied to even long-segment (TASC type D) occlusions extending for the length of the iliac arteries. In no other vascular territory has the shift from open surgery to interventional treatment been more apparent than in the AI segment. Using the Nationwide Inpatient Sample, one report documented an 850% increase in the use of percutaneous transluminal angioplasty and stenting for AI occlusive disease from 1996 to 2000, along with a simultaneous decrease of 16% in the rate of aortobifemoral grafting.34 Further reflecting this transformation in management strategy, in anatomically favorable circumstances, concomitant renal or mesenteric artery stenosis might be treated percutaneously as a staged initial procedure, even when subsequent open surgical AI reconstruction is planned.35 Decision making for surgical versus endovascular treatment is discussed in detail in Chapter 109.
In the current era, in which direct reconstruction for AI disease has been relegated to a second- or even third-line therapy, open bypass is increasingly undertaken in patients in whom endovascular treatment has been technically unsuccessful or in those with such extensive disease that an endovascular approach is deemed inadvisable. These shifting demographics have been reflected in reports documenting the increasing complexity of aortobifemoral grafting, with a higher incidence of suprarenal clamping, adjunctive visceral revascularization, simultaneous operative inflow and outflow disease, and repeated grafting.4,36 Patients with a combination of more proximal aneurysmal disease and common or external iliac occlusive disease continue to be good candidates for open reconstruction, although this group will continue to diminish with ongoing technologic advancements. Patients with extensive calcification at the aortic bifurcation thought to be at risk for rupture with balloon angioplasty and those with disease extending to the CFA are additional examples of patients who were once but are no longer considered unsuitable for an endovascular approach. The incidence of rupture has proved low in the former group, and the introduction of low-profile covered stents and techniques of primary stenting is likely to further increase the safety of endovascular treatment in this clinical setting. The second subgroup of patients can potentially be managed with a hybrid approach, whereby the CFA plaque is treated with a traditional endarterectomy and patch repair and the iliac component is concurrently addressed with endovascular techniques.37
Patients with early recurrence of AI disease after angioplasty or stenting represent a growing group for whom open surgical repair may be indicated. Patients with significant renal failure in whom endovascular therapy entails a prohibitive risk of triggering dialysis dependence are also considered better suited for operative repair. Complications of endovascular treatment, including dissection and vessel rupture, are infrequent indications for surgical reconstruction. At the advent of the endovascular era, there were significant fears that endoluminal therapy would accelerate the disease process or adversely affect available surgical options. Although these concerns have not been realized to any great extent in clinical practice to date, there are no doubt cases in which focal aortic disease has been rendered unsuitable for subsequent endarterectomy after stent placement. Similarly, some degree of stent explantation may be necessary to successfully complete aortofemoral reconstruction in patients who have previously undergone stent placement to the level of the renal arteries or in those who imprudently had stenting of their CFAs or profunda femoris arteries.
Claudication
The traditional indications for surgical reconstruction for symptomatic AI occlusive disease are disabling claudication, ischemic rest pain, and tissue loss. Claudication is a relative indication for intervention, given the natural history of the disease. Multiple reports, among them the Framingham Heart Study, indicate that patients with claudication have increased rates of cardiovascular mortality but an overall low risk of associated limb loss.5,38 The majority of claudicants demonstrate a stable pattern of disease throughout their lifetimes or have an improvement in symptoms as a result of risk factor modification, whereas 20% to 30% require operation within 5 years as a result of disease progression. The annual rates of mortality and limb loss in patients with claudication have historically been reported as 5% and 1%, respectively.39 Of relevance to a discussion of the appropriate threshold for intervention for claudication, Rutherford has taken issue with these commonly quoted estimates. Citing evidence based on objective hemodynamic confirmation of the diagnosis rather than clinical assessment alone, he believes that the actual annual rate of limb loss for claudicants is closer to 5%, particularly in those with ankle-brachial indices in the range of 0.4 to 0.6.40 Others have offered evidence that claudicants with AI disease are more likely to progress to CLI.41
In any case, the degree of disability that a particular level of claudication represents remains a subjective assessment by both the patient and the surgeon. For example, two-block claudication in a younger patient whose livelihood depends on walking tolerance constitutes a more significant disability than the same degree of claudication in an older, retired individual who can attend to his or her daily affairs without significant consequence. The anticipated benefit of therapy—that is, improvement in functional quality of life—must be carefully weighed against the specific limitations imposed by concomitant cardiac impairment, pulmonary disease, and musculoskeletal conditions in a given patient.
As the associated risks of AI balloon angioplasty and stenting have fallen and the relative success rates have risen in recent years, the threshold for offering endovascular treatment to patients with claudication at all points along the spectrum of occlusive disease has significantly decreased. Indeed, patients once considered appropriate only for risk factor modification, exercise therapy, and medical treatment are now increasingly being offered percutaneous revascularization as a primary treatment option. As a corollary trend, balloon technology is increasingly being applied to hypogastric arterial disease, primarily for buttock or hip claudication, to a degree not previously seen and to a degree not paralleled by an increase in surgical revascularization.42
The indications to proceed to surgical reconstruction for claudication have not shifted appreciably during this same period. Given the relatively benign natural history of the disease, a conservative treatment approach continues to be appropriate for many patients with claudication. Unlike the scenario in patients with CLI, it has been argued that an overly aggressive approach might place patients without limb-threatening conditions at unnecessary risk for adverse outcomes. The majority of claudicants remain stable for years, allowing time for collateral pathways to develop; this often results in sufficient improvement, making intervention unnecessary.43 In contrast, for patients who are good surgical risks and have disease limited to the aortobifemoral segment, surgical bypass is an appropriate option. With current levels of perioperative morbidity and mortality, bypass grafting can be undertaken safely and with the expectation of excellent long-term patency rates.
Critical Limb Ischemia
CLI is associated with likely amputation and significant suffering unless revascularization is undertaken. As such, the presence of ischemic rest pain, frank ulceration, or digital gangrene is a well-accepted and unambiguous indication for surgical correction of AI disease. As mentioned, patients manifesting pregangrenous or gangrenous skin changes typically have hemodynamically significant infrainguinal as well as AI occlusive disease.12 Thus, whereas patients treated for claudication or rest pain usually require only a single-stage inflow operation, simultaneous or staged inflow and outflow revascularization should be considered in patients with tissue loss (see under Multilevel Occlusive Disease).
Patients with an aortic or iliac source of distal emboli, typically from an ulcerated atheromatous plaque or so-called shaggy aorta, represent another group in which operative reconstruction is clearly indicated.44 Such patients may be without symptoms of claudication, and the culprit lesion or lesions may be hemodynamically insignificant. In these cases, the goal of intervention is usually not to relieve obstruction but to prevent recurrent distal embolization.
Studies have documented the generally excellent outcomes of aortobifemoral grafting in elderly patients, suggesting that in older patients who are otherwise good surgical risks, surgical therapy should not be withheld.45 Conversely, lower long-term patency rates with aortic bypass in younger patients or those with smaller aortic diameters suggest that caution should be applied to early surgical intervention in these patient populations.45
Surgical Treatment
The modern therapeutic armamentarium for treatment of arterial occlusive diseases is barely half a century old. Its development has been marked by several landmark advances, among them the discovery of the anticoagulant heparin by Best in the early 1930s, the advent of arteriography in 1927, the first application of arterial homografts by Gross in 1948 and prosthetic grafts by Voorhees in 1952, and the introduction of balloon angioplasty by Gruntzig in 1974 and of stenting by Puel and Sigwart in 1986. Continual improvements in surgical instrumentation and suture materials to facilitate vascular reconstruction and general advances in perioperative management, including cardiopulmonary care, infection control, and blood product use, have further contributed to the dramatic progress made. Historically, the surgical options for AI occlusive disease have included AI endarterectomy, aortobiiliac bypass, ABFB, and extra-anatomic bypass in the form of iliofemoral, femorofemoral, or axillofemoral grafting (see Chapter 111). Given its superior long-term patency rates, aortobifemoral grafting is currently considered the revascularization procedure of choice unless the patient is a poor candidate for major open surgery.
Preoperative Preparation
Careful assessment of the factors that influence operative risk and postoperative complications is an important component of the surgical management of aortic occlusive disease. Patient selection should be based on an objective evaluation of the patient’s comorbidities, functional limitations, and life expectancy. Particular attention should be paid to those patients with the identified risk factors of cardiac, renal, or pulmonary disease (see Chapters 39, 40, and 41). The patient’s cerebrovascular, hepatic, and hematologic status should also be reviewed, with further attention given to those with a personal or family history of hypercoagulability (see Chapter 38).
In patients with known renal insufficiency, elective AI surgery should not immediately follow diagnostic angiography; if possible, it should be delayed to allow recovery from the nephrotoxic effects of the contrast load. Similarly, preoperative fluid status should be optimized to the extent possible, with the avoidance of extremes of hypovolemia or fluid overload to minimize the risk of further postoperative renal compromise or congestive heart failure. This is particularly important if concomitant renal revascularization is being considered, which necessitates a period of renal ischemia and confers additional risk in the form of potential emboli or perioperative renal hypoperfusion. Patients with significant pulmonary dysfunction can usually be identified by history and physical examination, making routine pulmonary function testing unnecessary. If compromised pulmonary reserve is present, an aggressive regimen of preoperative pulmonary rehabilitation in the form of steroid and bronchodilator therapy may be beneficial to optimize the perioperative status.46 Smoking cessation is critical, and preoperative weight loss in obese patients is of further value in this regard. Perioperative pulmonary risk can also be reduced by the use of postoperative epidural analgesia and by strict adherence to protocols aimed at preventing postoperative deep venous thrombosis and pulmonary embolism.
Coronary Artery Disease
Nearly half of patients proceeding to surgery for AI occlusive disease have significant coronary artery disease.47 The reduced perioperative mortality and morbidity seen with AI surgery in recent years is largely due to advances in the management of concomitant coronary disease (see Chapter 39). Specifically, better preoperative identification of the small number of patients in need of initial coronary revascularization, awareness of the benefit of a waiting period between coronary stenting and major noncoronary vascular surgery, improved perioperative pharmacologic management of patients with impaired myocardium, and more focused efforts to tailor operative and postoperative fluid administration to the individual patient’s myocardial reserve are all well-recognized advances.48,49 Nevertheless, myocardial infarction remains the leading perioperative and long-term complication of AI surgery. For this reason, much energy has been devoted to identifying patients at particular risk for adverse coronary events stemming from the stress of surgery.50 The American Heart Association has recently updated its consensus recommendations regarding preoperative cardiac assessment in patients with peripheral arterial disease, reflecting the latest investigative results.50 Although the trend has clearly swung toward less extensive preoperative cardiac evaluation, it is not unreasonable to assume that all patients undergoing AI reconstructive surgery have some degree of coronary disease and would likely benefit from basic preventive measures. For instance, the routine use of perioperative beta blockade in high risk patients is probably the most important practice (see Chapter 39).51 Although the administration of aspirin was routinely discontinued in the past, the cardioprotective value of continuing aspirin through the time of AI reconstruction has now been clearly documented.52 In contrast, although lower extremity, carotid, and even coronary revascularization is increasingly being performed without discontinuation of clopidogrel, its use is still thought to be prohibitive in major cardiovascular surgery, given the associated bleeding risk.50,53 The use of mechanical bowel preparations and bowel-sterilizing oral antibiotics was previously common practice, but this is no longer routine. Finally, all patients should be administered appropriate preoperative intravenous antibiotics, typically either cefazolin or vancomycin, to ensure adequate tissue levels at the time of the skin incision.
Aortoiliac Endarterectomy
dos Santos’s successful endarterectomy of an atherosclerotic CFA lesion was serendipitous, in that he had not originally intended to remove any intima or media. His success, after multiple failed attempts, was attributed to the novel use of heparin.2 Wylie et al3 soon extended this technique to the AI level, and during the 1950s and 1960s, endarterectomy was the standard therapy for severe AI occlusive disease. Enthusiasm for the procedure dimmed, however, with the introduction of prosthetic graft material 10 years later, which was in turn largely replaced by aortic bypass grafting.
Patient Selection
An obvious benefit of endarterectomy is the elimination of the need for a prosthetic graft, making it an appealing alternative in the setting of infection and removing the possibility of myriad late graft-related complications. Advocates have likewise pointed to the advantages of endarterectomy for younger patients or those with small vessels who are less than ideal candidates for endovascular therapy or aortobifemoral grafting.54 Patients with erectile dysfunction attributable to proximal segment hypogastric occlusive disease are also well suited to this therapeutic option. Such patients can be expected to have a greater degree of improved pelvic perfusion after thorough endarterectomy of their AI and hypogastric segments compared with those undergoing ABFB grafting, and high rates of restored sexual function have been reported.54,55
Endarterectomy is most feasible and durable when it is applied to focal stenotic lesions in large-caliber, high-flow vessels. Indeed, the technique has proved particularly efficacious in patients with localized disease limited to the distal aorta or proximal iliac arteries (see Figs. 110-4 and 110-7), and excellent long-term patency rates, on a par with aortic bypass grafting, have been reported.54,56–58 In distinction, results in cases of long-segment disease involving the entire infrarenal aorta and extending into the external iliac arteries have been disappointing.59 In current practice, endarterectomy is now rarely performed for AI disease, having fallen into disfavor owing to its relative technical difficulty, potential for significant blood loss, and poor durability as well as the clear advantages of bypass grafting in this location. The increasing popularity of endovascular therapy is further eroding the already small proportion of patients considered suitable for this reconstructive approach. As a result, the number of vascular surgeons who are comfortable and facile with this technique is limited; those who maintain their familiarity with endarterectomy and appreciate its benefits will be best positioned to serve the needs of their patients.
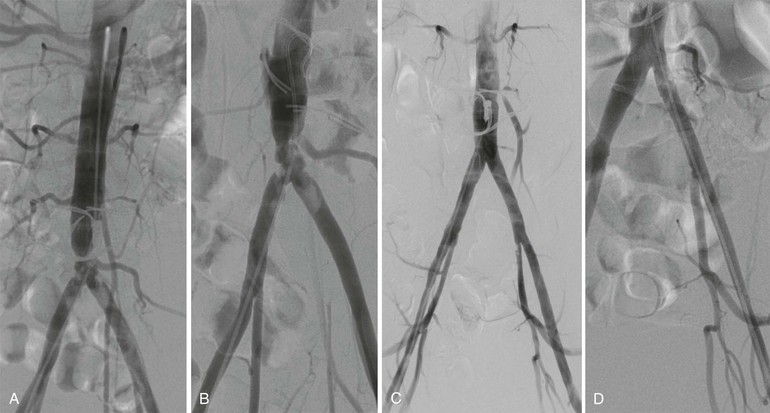
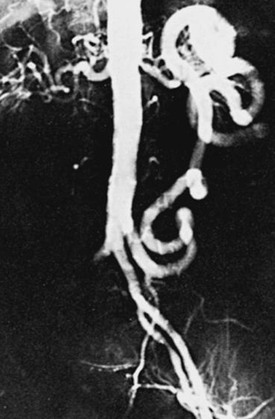
Figure 110-7 A and B, Anteroposterior (A) and left anterior oblique (B) angiographic images of bulky calcific atherosclerotic disease limited to distal aorta and proximal common iliac arteries, amenable to aortoiliac endarterectomy. Note the hypertrophic inferior mesenteric artery and iliolumbar collateral vessels. C, Completion angiogram after distal aortic and bilateral common iliac artery endarterectomy and Dacron patch angioplasty. D, Continued durability of result 3 years postoperatively.
Technique
Endarterectomy is a direct disobliterative, debulking technique that takes advantage of the pathologic localization of atherosclerosis to the intima and media. Typically, a cleavage plane is easily developed between the plaque and the outer layers of the vessel wall, with the exact location dependent on the size, location, and muscle content of the involved artery. Haimovici60 has characterized the three cleavage planes encountered in the operative setting as subintimal, transmedial, and subadventitial; in his view, the last two planes are preferable because the subintimal plane predisposes to subsequent thrombosis. The residual outer layer is generally of sufficient mechanical strength to hold surgical sutures and to resist disruption or progressive enlargement when it is newly subjected to arterial pressure. In atypical circumstances, the residual adventitia may be attenuated to such a degree that reconstruction of the wall with a patch or, in extreme cases, an interposition graft proves necessary. In practice, this most often occurs when the plaque is extensively calcified.
The various techniques all involve blunt separation of the plaque, termination by spontaneous tapering or sharp division, and careful attention to the endpoints (Figs. 110-7 and 110-8). Tacking sutures should be used when necessary, particularly to secure the distal endpoint, which must be firmly adherent to resist dissection or flap elevation leading to thrombosis. The simplest approach from a technical standpoint is the so-called open method, which employs a longitudinal arteriotomy that allows direct visualization of both endpoints as well as the entire endarterectomized surface; this technique is most commonly used for disease limited to the aorta and common iliac arteries. If primary closure is rendered problematic by the small or marginal caliber of the vessel, patch angioplasty with use of a vein, synthetic material, or bovine pericardial patch material should be undertaken.
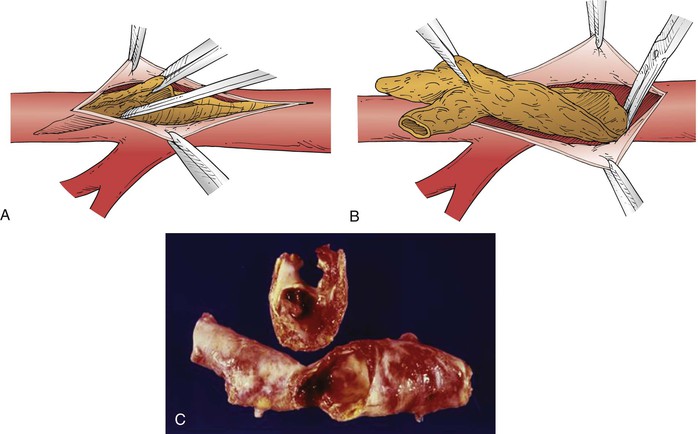
Figure 110-8 A, The technique of endarterectomy involves the initial separation of plaque in the appropriate cleavage plane, with mobilization facilitated by a fine spatula. B, Ideally, the endarterectomy is terminated by feathering to a tapered endpoint. C, Photograph of an operative specimen removed by endarterectomy.
Extraction, eversion, and semiclosed methods are variations that can be helpful in specific anatomic situations. The last technique, useful to treat the external iliac arteries, involves arteriotomies at both the proximal and distal extents of the plaque and the initiation of plaque excision at the endpoints, similar to that undertaken in the open technique. A ringed stripper is then advanced between the endpoints to complete the plaque disengagement, obviating the need for full exposure of the treated segment. In the early 1970s, Inahara61 modified the technique of eversion endarterectomy first described by Harrison et al,62 whereby the circumferentially dissected iliofemoral arterial segment is transected distally and passed proximally under the inguinal ligament into the pelvis before being endarterectomized and later reattached.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree