Fig 1 a–c.
Rupturing thoracic aortic aneurysm. a Unenhanced computed tomography (CT) demonstrates a high-attenuation hematoma in the right pleural space and middle mediastinum. There is an aneurysm of the descending aorta. b Unenhanced CT section 5 cm inferior to (a), reveals an intramural hematoma (IMH) at the inferior margin of the aneurysm, with extravasation of blood into the middle mediastinum. Note the displacement of intimai calcium along the inner wall of the IMH. c Following the administration of intravenous contrast material, the IMH is harder to visualize because of the wider window used to display the CT angiogram.
There are two limitations to this traditional classification. The first concerns the omission of a rupturing true aortic aneurysm, as the nature of the presentation and the severity of the event are similar in PAU and the other acute aortic syndromes. The second limitation is that IMH, defined as a stagnant intramural collection of blood, can be observed in the setting of AD, PAU, and rupturing aortic aneurysm. As such, it is a feature or characteristic associated with any of the acute aortic syndromes, reflecting degradation of the aortic wall as a harbinger of impending aortic rupture.
In consideration of these two points, a new classification scheme has been proposed based upon the primary location of the lesion within the aortic wall. In this new classification scheme, there are three pathological entities: AD, PAU, and rupturing aortic aneurysm [5]. These three entities are differentiated by the fact that AD principally involves the aortic media, PAU originates within the aortic intima, and aortic aneurysm is a disease of all three layers. The presence of IMH is an observation or epi-phenomenon to be applied to any of these three fundamental pathologies. In the setting of an isolated IMH without PAU, “non-communicating dissection” has been proposed as a descriptor, although the term “IMH” is more commonly associated with this lesion.
Essential Elements of Aortic Imaging Reports
In 2010, a group of medical organizations representing the disciplines of cardiology, radiology, thoracic surgery, and anesthesia published guidelines for the diagnosis and management of patients with thoracic aortic diseases. In this report, the authors identified eight essential elements that should be addressed in aortic imaging reports [2]. While these guidelines are not comprehensive, nor do they imply the necessity of reporting every element in every case, they are a useful construct from which to build any formalized description of the imaging findings of an acute aortic syndrome. They are the following.
1.
The location at which the aorta is abnormal.
2.
The maximum diameter of any dilation, measured from the external wall of the aorta, perpendicular to the axis of flow, and the length of the aorta that is abnormal.
3.
For patients with genetic syndromes who are at risk for aortic root disease, measurements of the aortic valve, sinuses of Valsalva, the sinotubular junction, and the ascending aorta.
4.
The presence of internal filling defects consistent with thrombus or atheroma.
5.
The presence of IMH, PAU, or calcification.
6.
Extension of the aortic abnormality to include inter-branch vessels, including dissection and aneurysm, and secondary evidence of end-organ injury (e.g., renal or bowel hypoperfusion).
7.
Evidence of aortic rupture, including periaortic and mediastinal hematoma, pericardial and pleural fluid and contrast extravasation from the aortic lumen (Fig. 2).
8.
When a prior examination is available, direct image-to-image comparison to determine whether there has been any increase in lesion diameter.
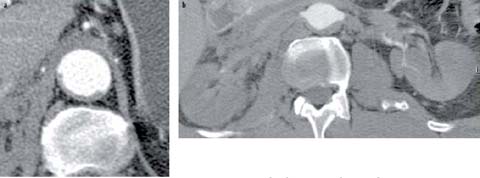
Fig 2 a, b.
Type B aortic dissection. a The true lumen is completely collapsed posteriorly and only the true lumen fills with contrast material. The entry tear was (not shown) in the proximal descending aorta. b Because of their supply from the aortic true lumen, the renal arteries do not opacity and the kidneys are not perfused
CT-Based Imaging Approaches to Acute Aortic Syndromes
High-quality and comprehensive aortic and end-organ assessment is performed using multi-detector row CT with at least 16 detector rows. This scanner configuration allows for imaging from the neck through the pelvis, acquiring ≽1.5-mm-thick transverse sections during the arterial phase of enhancement from an intravenous contrast administration. It also allows for the use of electrocardiographic (ECG) gating of the scan when appropriate, as described below.
Unenhanced CT
An unenhanced scan prior to the administration of intravenous contrast can be valuable for the detection of intramural and periaortic blood which is often subtle. It can also be useful for mapping the specific regions of the aorta that are abnormal and thus guide the mode of subsequent CT angiographic acquisition. While an associated increase in radiation exposure results from this approach, the potential value of the information almost always outweighs the risk. Using dual-energy scanning, a virtual unenhanced scan might obviate the need for a separate unenhanced acquisition. However, this approach has not been comprehensively validated in acute aortic syndromes. Moreover, it eliminates the possibility of using the preliminary unenhanced acquisition to guide the decision to gate the CT angiogram.
CT Angiography
While unenhanced imaging can reveal aortic dilation, intramural and extra-aortic hemorrhage, and in uncommon circumstances directly visualize an intimai flap, the use of intravenously administered contrast medium is required for a complete assessment in patients in whom acute aortic syndrome is suspected (Fig. 3). The volume and flow rate of the contrast material should be adjusted based on patient size. A concentrated iodine solution containing ≾350 mg of iodine/mL will assure adequate intravenous delivery of iodine with a safe and reliable flow rate of the contrast material into the peripheral vein. Typical volumes and injector flow rates for iodinated contrast range between 60 and 115 mL at flow rates between 3.5 and 6 mL/s.
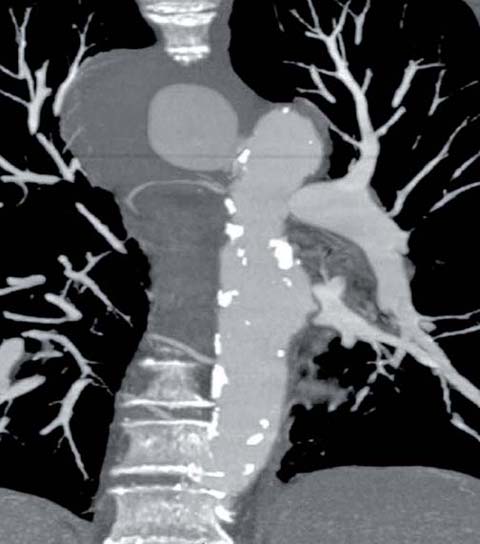
Fig 3.
Penetrating atherosclerotic ulcer with pseudoaneurysm formation in the proximal descending aorta
To assure diagnostic aortic enhancement throughout the CT acquisition, the duration of the contrast injection should exceed the scan duration by 5–10 s, and initiation of the CT angiographic acquisition should be based on the active monitoring of the arrival of iodine within the descending thoracic aorta.
Imaging the Abdominal Aorta and Iliac Arteries
Because of the likelihood of direct extension of thoracic aortic disease into the abdominal aorta and iliac arteries, the presence of unrelated but important abdominal aortoiliac pathology, the value of assessing the caliber of a transfemoral delivery route to intra-aortic repair devices, and the possibility of abdominal visceral ischemia, scan ranges that extend through the abdomen and pelvis are highly recommended as a routine approach to imaging acute aortic syndromes. By beginning the scan in the neck and extending inferiorly below the lesser trochanters of the femurs, the scan range will comfortably include several centimeters of the cervical carotid arteries through the bifurcation of the femoral artery. Scan ranges that include less anatomy risk the possibility that important observations will be missed such that additional CT scans with further injections of iodinated contrast material may be required.
Electrocardiographic Gating
When the ascending aorta is involved by an acute aortic syndrome, electrocardiographic (ECG) gating can be valuable. ECG gating allows for clear delineation of the position of the intimai flap across the cardiac period, distinction of the involvement of the structures of the aortic root including the coronary artery ostia and the aortic annulus, elimination of pulsation-related artifacts that can blur the aortic wall, and subtle regions of extravasation. Unlike the use of ECG gating in the setting of coronary artery disease assessment, the strategy for using ECG gating in acute aortic syndromes does not rely upon the manipulation of heart rate or coronary artery dimension using β-blockers or nitrates. Regardless of the basal heart rate, the placement of ECG leads and the acquisition of a retrospectively gated CT scan (with judicious use of ECG directed X-ray tube current pulsing to minimize radiation exposure) allow for a four-dimensional assessment of the aortic root, aortic valve, coronary arteries, and ascending aorta. It is sufficient to reconstruct 10 phases every 10% of the R-R interval. Gating is only beneficial through the thoracic aorta. The abdomen and pelvis are imaged after the chest acquisitions, using a non-gated acquisition with a minimization of the delay between the two scans so that only one contrast injection is required.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


