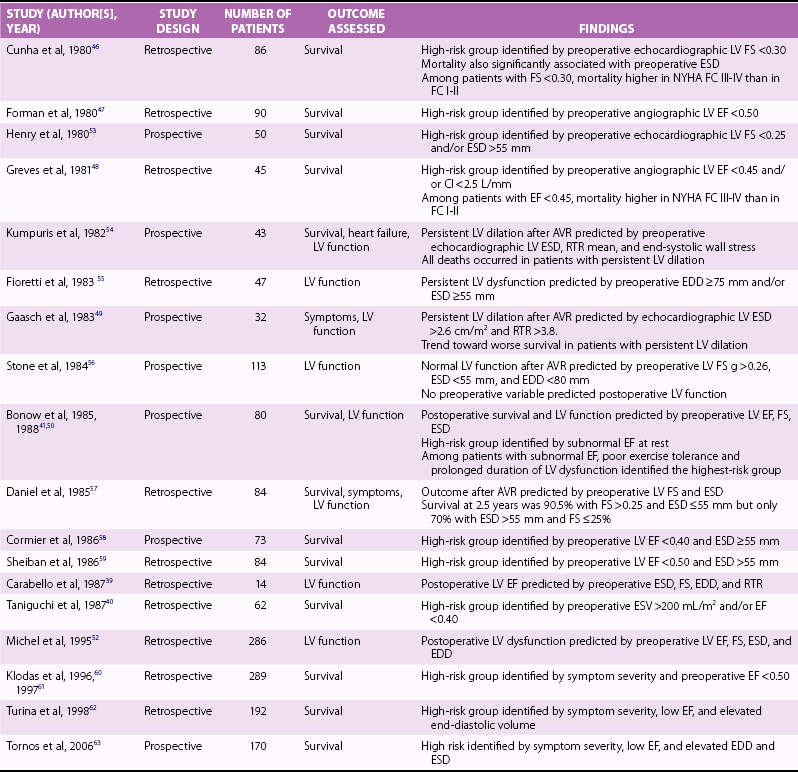
Chapter 12 Pure aortic regurgitation (AR) has multiple causes involving abnormalities of the aortic valve leaflets, aortic root, or both ( Table 12-1). The most frequent causes are congenital abnormalities of the aortic valve (most notably bicuspid valves [ Figure 12-1] but also unicuspid, tricuspid, and quadricuspid valves), rheumatic disease, infective endocarditis, calcific degeneration, and myxomatous degeneration. Other common causes of AR represent diseases of the aorta without direct involvement of the aortic valve, as in ascending aorta dilation secondary to atherosclerosis or systemic hypertension, idiopathic annuloaortic ectasia, aortic dissection, and Marfan syndrome.1,2 Less common causes of AR include traumatic injuries to the aortic valve, aortitis occurring in ankylosing spondylitis, syphilitic infection, rheumatoid arthritis, osteogenesis imperfecta, giant cell aortitis, Takayasu disease, Ehlers-Danlos syndrome and Reiter syndrome. AR can also occur in cases of discrete subaortic stenosis and ventricular septal defect with prolapse of an aortic cusp, in ruptured aneurysms of the sinuses of Valsalva, and in cases of fenestrated aortic cusps. 3 AR has also been described as a complication of balloon aortic valvuloplasty and transcatheter aortic valve implantation,4,5 and anorectic drugs and dopamine agonist have also been reported to cause AR.6,7 However, in many cases of AR the precise etiology is unclear. In a pathologic study of a surgical series of excised aortic valves, up to 34% cases of pure AR were considered of unclear etiology. 2 In the Euro Heart Survey on Valvular Disease, AR represented 13.3% of patients with single native left-sided disease: 15.2% of cases were considered of congenital origin, and the same percentage was observed for rheumatic origin. 8 TABLE 12-1 Causes of Aortic Regurgitation FIGURE 12-1 Role of echocardiography in the diagnosis of aortic regurgitation etiology. In acute severe AR the sudden large regurgitant volume is imposed on a left ventricle of normal size that has not had time to adjust to the volume overload. Thus, the acute increase in diastolic flow into the nondilated left ventricle leads to a marked elevation in end-diastolic pressure owing to a rightward shift along the normal LV diastolic pressure-volume curve. In severe cases, the increased ventricular pressures during the diastolic filling period in conjunction with the decrease in the aortic diastolic pressure leads to a rapid equalization of aortic and LV pressures at end-diastole 9 ( Figure 12-2). FIGURE 12-2 Continuous-wave Doppler curves. With acute regurgitation, forward cardiac output is decreased because the total stroke volume of the nondilated ventricle now includes both regurgitant and forward stroke volumes. Compensatory tachycardia may partially correct this decline in forward stroke volume, but it is often insufficient to maintain cardiac output, and hence patients may be in cardiogenic shock. Pulmonary edema results from the markedly elevated LV end-diastolic pressure and concomitant elevation of pulmonary venous pressure. In addition, coronary flow reserve is acutely diminished, possibly leading to subendocardial ischemia. As the LV end-diastolic pressure approaches the diastolic aortic and coronary artery pressures, myocardial perfusion pressure in the subendocardium is diminished, whereas myocardial oxygen demand is increased by the effects of greater afterload and tachycardia. 10 Many of the characteristic physical findings in chronic volume overload are modified or absent when valvular regurgitation is acute. Therefore, the severity of AR can be underestimated. Because of the acute hemodynamic deterioration, patients with acute AR are often tachycardic and tachypneic and have pulmonary edema. However, LV size may be normal on physical examination, and chest radiography may not demonstrate cardiomegaly. In addition, pulse pressure may not be increased because systolic pressure is reduced in relation to the decrease in forward stroke volume and because diastolic pressure equilibrates with the elevated LV diastolic pressure. In the absence of a widened pulse pressure, the characteristic peripheral signs of AR are absent. Although a diastolic murmur is usually present, it can be soft and short because the rapidly rising LV diastolic pressure reduces the aortic-ventricular pressure gradient. The murmur is thus often poorly heard. 11 Echocardiography is indispensable in confirming the presence and severity of AR, assessing its cause, and determining whether there is a rapid equilibration of aortic and LV diastolic pressure. Evidence for rapid pressure equilibration includes a short AR diastolic half-time (<300 ms) (see Figure 12-2), a short mitral deceleration time (<150 ms), and premature closure of the mitral valve ( Figure 12-3). FIGURE 12-3 Transthoracic parasternal long-axis view echocardiogram in a patient with acute aortic regurgitation due to infective endocarditis. Transesophageal echocardiography is indicated when aortic dissection ( Figure 12-4), acute endocarditis ( Figure 12-5), or trauma is suspected or when the mechanism of acute AR is uncertain. Computed tomography (CT) or cardiac magnetic resonance imaging (CMR) can be used in some settings if it will lead to a more rapid diagnosis than can be achieved by transesophageal echocardiography.12–14 FIGURE 12-4 Acute aortic regurgitation in a patient with ascending aorta dissection. FIGURE 12-5 Transesophageal echocardiography in a patient with infective endocarditis. Death due to pulmonary edema, ventricular arrhythmias, electromechanical dissociation, or circulatory collapse is common in acute, severe AR. Thus, patients require emergency or urgent surgery for correction of the underlying disease process and relief of the acute volume overload. Intraaortic balloon counterpulsation is contraindicated. In patients with acute AR due to an ascending aortic dissection, prompt surgical intervention is needed, including a composite replacement of the aorta along with aortic valve or a valve-sparing reimplantation technique.15–17 Patients with severe acute AR due to infective endocarditis need immediate initiation of antibiotics and aggressive medical treatment. If the hemodynamic situation does not immediately improve, emergency valve replacement may be life saving. If the clinical situation stabilizes, surgery can be postponed for a few days with the patient under strict medical supervision in order to allow antibiotic treatment to become effective before surgical correction.18–20 The left ventricle responds to the volume load of chronic AR with a series of compensatory mechanisms, including an increase in end-diastolic volume, an increase in chamber compliance that accommodates the increased volume without a rise in filling pressures, and a combination of eccentric and concentric hypertrophy. The central hemodynamic feature of chronic AR is combined volume and pressure overload of the left ventricle.21–23 Because total LV stroke volume equals forward plus regurgitant stroke volumes, normal cardiac output is maintained by an increase in total stroke volume corresponding to the severity of regurgitation. This increase in total stroke volume is achieved by progressive ventricular dilation, with increased end-diastolic and end-systolic volumes. The greater diastolic volume permits the ventricle to eject a large total stroke volume, thus keeping forward stroke volume in the normal range. This is accomplished through rearrangement of myocardial fibers with the addition of new sarcomeres and development of eccentric LV hypertrophy. 24 As a result, preload at the sarcomere level remains normal or near normal and the ventricle retains its preload reserve. The enhanced total stroke volume is achieved through normal performance of each contractile unit along the enlarged circumference. 25 Thus LV ejection performance is normal, and ejection phase indices such as ejection fraction (EF) and fractional shortening remain in the normal range. However, the enlarged chamber size and the associated increase in systolic wall stress also result in a stimulus for further hypertrophy. 26 Despite an increase in end-systolic dimension and pressure early in the course of the disease, end-systolic wall stress is maintained in the normal range by a compensatory increase in wall thickness. Thus, patients with compensated chronic AR have substantial increases in LV mass as well as LV volumes, and EF and end-systolic elastance tend to be normal. As the disease progresses, recruitment of preload reserve and compensatory hypertrophy permit the left ventricle to maintain normal ejection performance despite the elevated afterload.22,27,28 The majority of patients remain asymptomatic during this compensated phase, which may last for decades. During this compensated phase, ejection phase indices of LV systolic function at rest are normal. It is recognized, however, that other indices of LV function may not be normal. It is further recognized that the transition to LV dysfunction represents a continuum and that no single hemodynamic measurement represents the absolute boundary between normal LV systolic function and LV systolic dysfunction. In a large subset of patients, the balance among afterload excess, preload reserve, and hypertrophy cannot be maintained indefinitely. Preload reserve may be exhausted and/or the hypertrophic response may be inadequate, 29 so that further increases in afterload result in a reduction in EF, first into the low-normal range and then below normal. Impaired contractility may also contribute to this process. Dyspnea often develops at this point in the natural history. In addition, diminished coronary blood flow reserve in the hypertrophied myocardium may result in exertional angina. 30 However, this transition may be more insidious, and it is possible for patients to remain asymptomatic even after severe LV dysfunction has developed. LV systolic dysfunction (defined as an EF below normal at rest) is initially a reversible phenomenon related predominantly to afterload excess, and full recovery of LV size and function is possible with aortic valve replacement (AVR).31–42 With time, during which the left ventricle develops progressive chamber enlargement and a more spherical geometry, depressed myocardial contractility predominates over excessive loading as the cause of progressive dysfunction. This process can progress to the extent that the full benefit of surgical correction of the regurgitant lesion, in terms of recovery of LV function and improved survival, can no longer be achieved. A number of studies have identified LV systolic function and end-systolic size as the most important determinants of survival and postoperative recovery of LV function in patients undergoing valve replacement for chronic AR.43–64 Studies of predictors of surgical outcome are listed in Table 12-2. TABLE 12-2 Preoperative Predictors of Surgical Outcome in Aortic Regurgitation Modified from Bonow R, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2006;114:e84–231. Among patients undergoing valve replacement for chronic AR with preoperative LV systolic dysfunction, several factors are associated with worse functional and survival results after the operation. They are listed in Table 12-3. Table 12-3 Factors Predictive of Reduced Postoperative Survival and Recovery of Left Ventricular (LV) Function in Patients with Aortic Regurgitation and Preoperative LV Systolic Dysfunction Severity of preoperative symptoms or reduced exercise tolerance Severity of depression of LV ejection fraction Duration of preoperative LV systolic dysfunction From Bonow R, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2006;114:e84–231. Many patients with AR are diagnosed before symptom onset, on the basis of the finding of a diastolic murmur on physical examination, the discovery of an enlarged cardiac silhouette on chest radiography, or evidence of LV hypertrophy on electrocardiography. The most common initial symptom in patients with chronic severe AR is exertional dyspnea, most likely due to an elevated LV end-diastolic pressure with exercise. 65 Because chronic AR has a slowly progressive course, the gradual decrease in exercise capacity may not be recognized as abnormal by the patient, and therefore very careful questioning is often needed to elicit evidence of a subtle decrease in functional status. In cases in which symptoms are doubtful or equivocal, exercise testing may be valuable in assessing functional capacity. In more advanced cases, with severe LV dysfunction, patients can have symptoms of overt heart failure, including dyspnea at rest, orthopnea, and pulmonary edema. The acute onset of heart failure symptoms can occur in patients with chronic disease as a result of an acute increase in the severity of regurgitation, for example, in patients with infective endocarditis or aortic dissection. Angina may occur, even in the absence of atherosclerotic coronary artery disease, because of a decreased myocardial perfusion pressure, increased myocardial oxygen demand, and a decreased ratio of coronary artery size to myocardial mass. Syncope or sudden death, although rare, can occur in AR. Sudden death has been reported in association with extreme degrees of LV dilation. 66 In patients with mild or moderate AR, the only finding on physical examination may be the diastolic murmur, but in many patients there is also a systolic outflow murmur related to the increased stroke volume, and often the systolic murmur is more apparent than the diastolic murmur. Most cases of severe AR are detectable through physical examination with the combination of cardiac murmur, widened pulse pressure on blood pressure measurement, and peripheral findings related to this widened pulse pressure ( Table 12-4). Classically in severe AR systolic arterial pressure is elevated and diastolic pressure is abnormally low, but the blood pressure may remain normal in many patients with severe AR. 67 The apical impulse is diffuse and hyperdynamic and is displaced laterally and inferiorly because of the LV dilation. The carotid pulse is bounding with a more rapid rate of pressure rise in early systole as well as an increase in the amplitude of the systolic pressure curve. A bisferiens carotid pulse may be present. 68 In very severe cases, the head may bob forward with each heart beat (De Musset sign). The classic peripheral signs of AR are present only in cases of severe and chronic regurgitation and reflect the increased pulse pressure. They include the water-hammer or collapsing pulse (Corrigan pulse), 69 systolic pulsation of the fingernail bed on gentle pressure (Quincke pulse), 70 and a systolic and diastolic bruit over the femoral arteries on gentle compression by the stethoscope (Duroziez sign), a manifestation of the reversal of flow in the descending aorta. TABLE 12-4 Chronic Compensated, Chronic Decompensated, and Acute Aortic Regurgitation From Otto CM, Aortic regurgitation. In: Otto CM, editor. Valvular heart disease. 2nd ed. Philadelphia: Saunders, 2004. A short midsystolic murmur related to the increased ejection rate and stroke volume may be audible at the base of the heart and transmitted to the carotid vessels. The aortic regurgitant murmur is one of high frequency that begins immediately after S2, continues to S1, and has a decrescendo intensity. With valve leaflet abnormalities, the murmur is best heard along the left sternal border in the third or fourth intercostal space, whereas with aortic root disease, a selective radiation along the right sternal border is common. 71 However, the diastolic murmur is often not appreciated on physical examination. In comparison with Doppler echocardiography and aortic angiography, the sensitivity of auscultation for detection of AR is 37% to 73%, and the specificity is 85% to 92%.72–74 The loudness of the murmur correlates with disease severity to some extent. 75 Another classic finding in patients with severe chronic AR is the Austin Flint murmur, a low-pitched middiastolic rumble that mimics the murmur of mitral stenosis. 76 Comparisons of Doppler echocardiographic findings with physical findings suggest that this diastolic murmur is related to the severity of AR, with a jet directed toward the anterior mitral leaflet or LV free wall causing vibrations appreciated on auscultation as a low-pitched diastolic rumble.77–79 The physical findings in acute AR differ from those in chronic regurgitation, in parallel with the different hemodynamics of acute and chronic disease (see Table 12-4). The electrocardiogram (ECG) findings in patients with AR include voltage criteria for LV hypertrophy and associated repolarization abnormalities. A strain pattern on the resting ECG correlates strongly with abnormal LV dimensions, mass, and wall stress.80–82 However, some cases of severe AR and pathologic LV hypertrophy do not meet ECG criteria for LV hypertrophy. 83 When the ECG is normal at rest, flat and/or downsloping ST depression may develop with exercise, even in the absence of coronary artery disease, and is associated with an increased LV systolic dimension. 84 Ventricular ectopic beats and nonsustained ventricular arrhythmias are also relatively common in AR, and have a significant correlation with LV hypertrophy and function. 85 The chest radiograph shows an enlarged silhouette due to LV dilation. Aortic root enlargement is also frequently present as a result of primary diseases of the aorta or of dilation secondary to the increased flow. Both evidence of LV hypertrophy on ECG and cardiac size on radiography have been shown to be predictors of outcome after valve replacement.86–90 However, neither the ECG nor the chest radiograph offers sufficiently precise data to be useful in clinical decision making or sequential follow-up of patients with AR. After the history and physical examination, echocardiography is the most important examination in patients with AR. Echocardiography is used to diagnose and estimate the severity of regurgitation using color Doppler flow imaging (vena contracta of regurgitant jet) ( Figure 12-6) and pulsed-wave tissue Doppler imaging (holodiastolic flow reversal in the descending thoracic and abdominal aorta)91,92 ( Figure 12-7). These indices are influenced by loading conditions and the compliance of the ascending aorta and the left ventricle. Quantitative Doppler echocardiography, using the continuity equation or analysis of proximal isovelocity surface area, is less sensitive to loading conditions 93 and provides measures of regurgitant volume, regurgitant fraction, and effective regurgitant orifice. The criteria for defining severe AR are shown in Table 12-5. TABLE 12-5 Criteria for the Definition of Severe Aortic Regurgitation Modified from Zoghbi WA, Enriquez-Sarano M, Foster E et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777–8. FIGURE 12-6 Assessing severity of aortic regurgitation. FIGURE 12-7 Assessing severity of aortic regurgitation. Echocardiography is also performed to identify the mechanisms of regurgitation, describe the valve anatomy, and determine the feasibility of valve repair. An important role of echocardiography is to provide precise and reproducible measures of LV dimensions, volumes, and systolic performance, and therefore it is the cornerstone for clinical decision making and serial follow-up in patients with chronic AR ( Figure 12-8). Indexing for body surface area (BSA) is especially recommended in women and in men of small body size.61,94 Although LV EF is the fundamental parameter for evaluating LV contractility, new parameters obtained by tissue Doppler imaging and strain rate imaging may be useful in patients with borderline EF measurements. Two studies have shown peak systolic wave velocity less than 9 cm/s at the mitral annulus to be a predictor of complications and to be related to LV contractile reserve.95,96 Serial echocardiographic evaluations of LV size and function should take into account the potential of confounding factors, such as interval changes in instrumentation, variability in recording and measuring the data, variability in loading condition, and physiologic variability. When a change is detected, it is prudent to repeat the examination to confirm the magnitude and direction of the change. Good-quality echocardiograms and data confirmation are essential before surgery can be recommended to asymptomatic patients. Echocardiography should also image the aorta at four different levels: annulus, sinuses of Valsalva, sinotubular junction, and ascending aorta ( Figure 12-9). Transesophageal echocardiography may be performed to better define the anatomy of the valve and ascending aorta, especially when aortic pathology is suspected or a valve-sparing intervention is considered. 97 Studies have also demonstrated the feasibility and accuracy of three-dimensional transthoracic echocardiography (3D TTE) in quantifying AR. 98 FIGURE 12-8 Left ventricular dilation in aortic regurgitation. FIGURE 12-9 Parasternal long-axis view echocardiogram in a patient with aortic regurgitation. In patients with indeterminant echocardiographic findings, CMR is a reliable tool for assessment of the severity of AR.98a,99 Magnetic resonance phase-contrast sequences perpendicular to the aortic valve allow accurate antegrade and retrograde blood flow measurements in the ascending aorta, 100 so that the severity of AR by calculation of regurgitant volume, peak velocity, and regurgitant fraction can be assessed.101,102 Additionally, cine CMR sequences, such as steady-state free precession techniques, permit visualization of the aortic valve in a chosen plane with excellent image quality. Cine CMR also aids in determination of the morphology of the valve, and valve area can be measured by planimetry methods101–106 ( Figure 12-10). Moreover, with the use of serial short axis slices of the left ventricle, it is possible to calculate LV volumes, mass, and EF very accurately. Several studies have shown that CMR is an excellent technique to monitor LV volumes and EF with a high degree of interobserver reproducibility (r = 0.96-0.99). 107 Finally, the use of contrast agents (gadolinium-DTPA) and different magnetic resonance angiography sequences or three-dimensional whole chest steady-state free precession sequences (without contrast agent) enables determination of the aortic root and ascending aortic anatomy and diameters. FIGURE 12-10 Cardiac magnetic resonance imaging showing a bicuspid aortic valve with aortic regurgitation and ascending aorta dilation.
Aortic Regurgitation
Etiology
Leaflet abnormalities
Rheumatic disease
Aortic valve sclerosis and calcification
Congenital abnormalities (bicuspid, unicuspid, and quadricuspid valves, and AR associated with discrete subaortic stenosis and ventricular septal defect)
Infective endocarditis
Myxomatous valve disease
Complicating balloon valvuloplasty and transcatheter aortic valve implantation
Rare causes (drugs, leaflet fenestration, irradiation, nonbacterial endocarditis, trauma)
Aortic root abnormalities
Chronic hypertension
Marfan syndrome
Annuloaortic ectasia
Aortic dissection
Ehlers-Danlos syndrome
Osteogenesis imperfecta
Atherosclerotic aneurysm
Syphilitic aortitis
Other systemic inflammatory disorders (giant cell aortitis, Takayasu disease, Reiter syndrome)
Combined valve and aortic root abnormalities
Bicuspid aortic valve
Ankylosing spondylitis
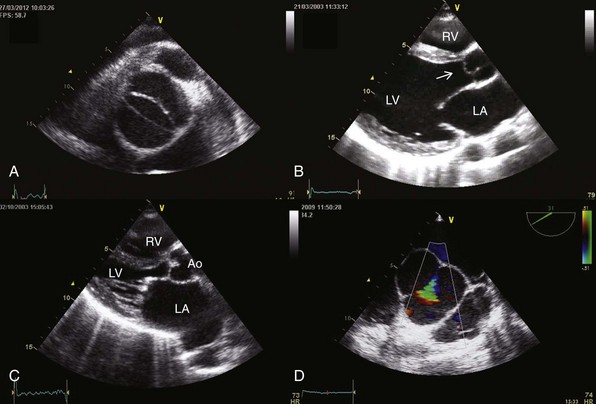
A, Transthoracic parasternal short-axis view showing a bicuspid aortic valve. B, Myxomatous aortic valve with a prolapse of the right coronary cusp (arrow). C, Rheumatic valvular disease with mitral and aortic involvement. D, Transesophageal echocardiography showing a central regurgitant orifice secondary to an annuloaortic ectasia defined by color Doppler (green triangular area) during diastole. Ao, aorta; LA, left atrium; LV, left ventricle; RV, right ventricle.
Acute Aortic Regurgitation
Pathophysiology
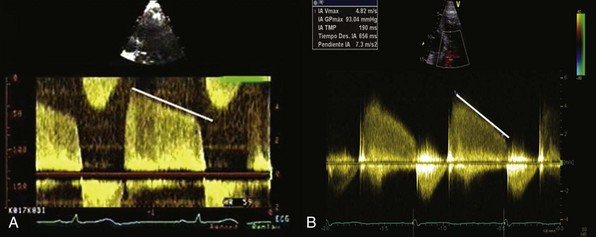
A, Chronic severe aortic regurgitation. B, Acute severe aortic regurgitation. Note the steeper deceleration slope in the acute case, which is due to the equalization of left ventricular and aortic diastolic pressures.
Diagnosis
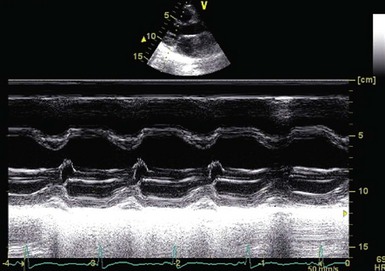
The M-mode images show early closure of the mitral valve.
A, Transesophageal echocardiography shows an intimal flap prolapsing through the aortic valve (arrow). B, Severe aortic regurgitation is observed by color Doppler (green area corresponds to regurgitant jet). Ao, aorta; LA, left atrium; LV, left ventricle.
A, Eversion of the left coronary cusp (large arrow) and vegetations in the left and right coronary sigmoids (small arrows). B, Jet of severe aortic regurgitation defined by color Doppler (green area). Ao, aorta; LA, left atrium; LV, left ventricle.
Management
Chronic Aortic Regurgitation
Pathophysiology
Clinical Presentation
Clinical History
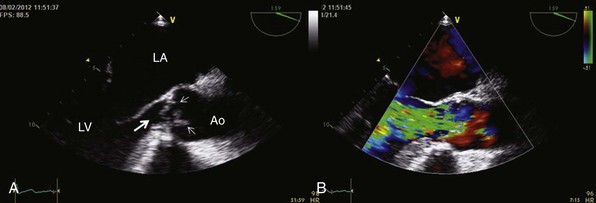
Physical Examination
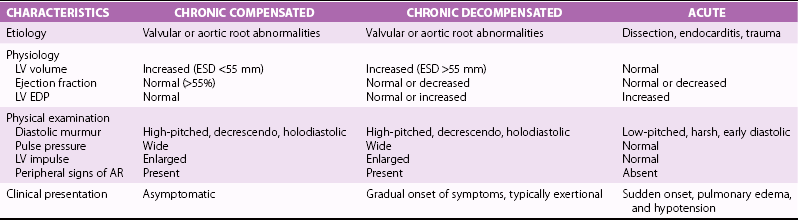
Electrocardiogram and Chest Radiography
Echocardiography
Specific signs
Central jet, width ≥ 65% of left ventricular outflow tract
Vena contracta > 0.6 cm
Supportive signs
Pressure half-time < 200 ms
Holodiastolic aortic flow reversal in descending aorta
Moderate or greater LV enlargement
Quantitative parameters
Regurgitant volume ≥ 60 mL/beat
Regurgitant fraction ≥ 50%
Effective regurgitant orifice area ≥ 0.30 cm2
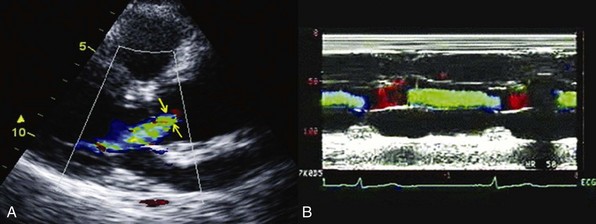
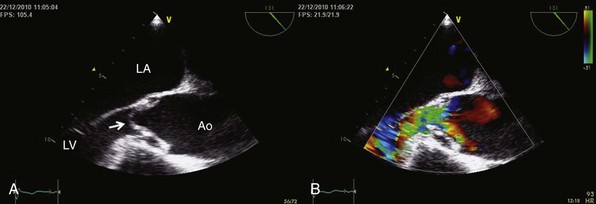
A, Parasternal long-axis view Doppler echocardiogram showing the vena contracta of the regurgitant flow in a patient with severe aortic regurgitation. B, Color M-mode echocardiogram in the same patient, showing the width of the regurgitant jet.
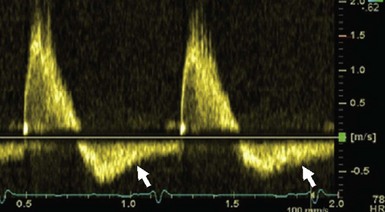
Pulsed Doppler echocardiogram in the abdominal aorta showing pandiastolic regurgitant flow (arrows).
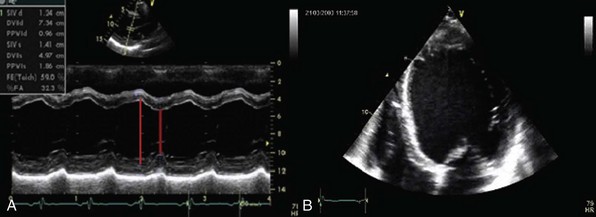
A, Transthoracic parasternal long-axis view echocardiogram showing enlargement of left ventricular parameters in a patient with severe chronic aortic regurgitation. B, Apical four-chamber view of the same patient showing a spherical enlargement of the left ventricle.
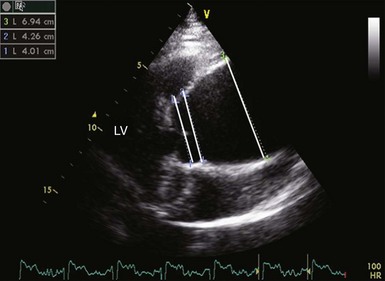
There is enlargement of the aortic root and ascending aorta. From left to right, lines identify the aortic diameter at the level of sinuses of Valsalva, sinotubular junction, and ascending aorta. LV, left ventricle.
Other Imaging Modalities
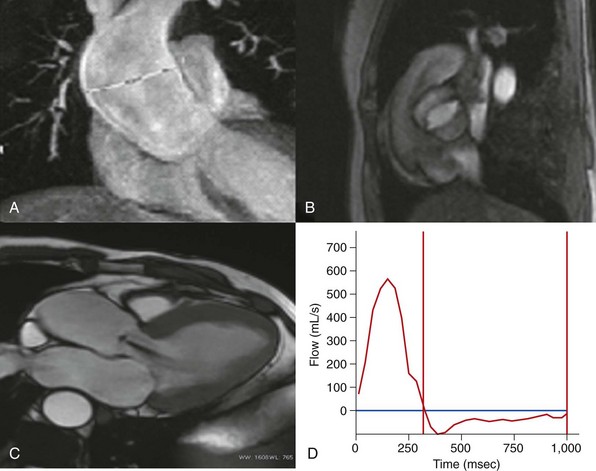
A, Fast single-shot steady-state free precession (SSFP) image in a coronal view. B, Retrospectively reconstructed magnitude image from a phase-contrast sequence showing a bicuspid aortic valve. C, Balanced SSFP image. Oblique axial left ventricle inflow/outflow view, showing grade 2 aortic regurgitation. D, Flow-versus-time plot for the ascending aorta. Antegrade flow calculated at 140 mL/beat, retrograde flow 40 mL/beat, and aortic regurgitant fraction 33%.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Aortic Regurgitation
Only gold members can continue reading. Log In or Register to continue





