Aortic Graft Infection with Aortoenteric Fistulae
JOHN B. LUKE and WILLIAM D. JORDAN Jr
Presentation
A 55-year-old male presented to the emergency department with worsening right foot pain for 2 weeks and short distance claudication. The patient had a history of occlusive disease including an aortobifemoral (AoBF) bypass 2 years prior. He also reported lethargy over the past month and intermittent fevers. He experienced one episode of hematemesis 1 week ago, but denied any blood per rectum. On exam, he had blood pressure of 130/85 and HR of 105. There were absent femoral pulses but adequate lower extremity perfusion with no ulcers. Ankle-brachial indices are 0.5 bilaterally. He has tenderness to palpation at epigastrium. Rectal exam identified occult blood.
Differential Diagnosis
In the patient presenting with an aortic graft and gastrointestinal (GI) bleed, a graft enteric fistula is not the most common cause of occult GI bleed; however, it is the most lethal. Thus, graft enteric fistula must be carefully considered for this patient. Although secondary aortoenteric fistula is rare, failure to promptly identify and treat this complication can be a fatal problem. Other common causes of GI bleed include peptic ulcer disease, gastritis, varices, colonic diverticulum, and malignancy. Aside from a GI bleed, other presenting symptoms include fever, lethargy, weight loss, abdominal/back pain, or a pulsatile mass. Lower extremity symptoms secondary to graft thrombosis may also exist.
Case Continued
The patient underwent further evaluation of his AoBF graft with CT angiography (Fig. 1), which reveals an occluded graft that is attached in an end-to-side configuration with periaortic inflammation and air present in the graft limbs. Laboratory values include a leukocyte count of 21 × 103 cc and hemoglobin of 9.2 g/dL.
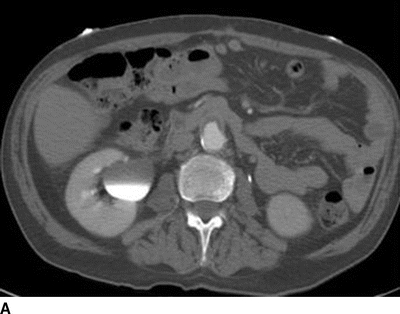
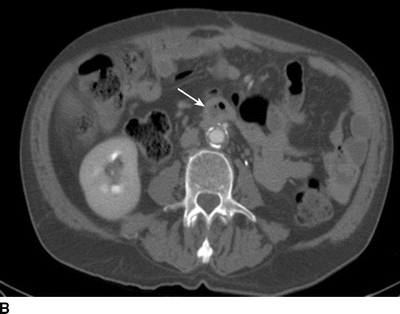
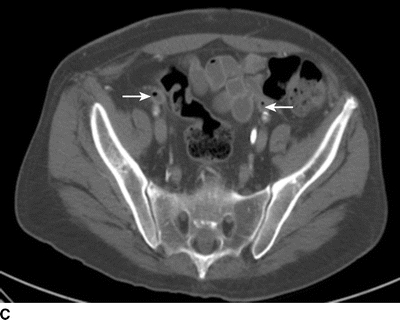
FIGURE 1 CT scan revealing (A) patent AoBF graft at proximal anastamosis with end-to-side configuration, (B) occluded main body AoBF graft with air in graft (arrow), (C) occluded graft limbs with air present (arrows).
Workup
In the hemodynamically unstable patient with high index of suspicion of graft enteric fistula, there may be limited time for a complete workup. Diagnosis is confirmed at time of emergency exploration.
In the stable patient, initial laboratory evaluation should include a leukocyte count along with differential. Baseline hematocrit is imperative when graft enteric fistula is suspected. ESR and CRP are nonspecific; however, they may be useful adjuncts to aid in the overall workup process. Further diagnostic tools should be considered based on the clinical condition of the patient.
The duodenum is the most common location of graft enteric fistula; thus, upper GI endoscopy should be performed early in the workup process. This will potentially diagnose a fistula with visualization of adherent clot or even graft in the duodenum. Endoscopy can also aid in ruling out items mentioned above in the differential diagnosis such as varices, gastritis, and peptic ulcer disease. It is important to examine the entire duodenum as a fistula will most commonly develop at the third or fourth portion. A smaller endoscope along with the patient’s peristalsis or double-balloon enteroscopy may be necessary for a complete evaluation. Extrinsic compression of the duodenum and pulsatility are clues that the examined portion is overlying the aorta. If an adherent clot is visualized in this area, it should not be disturbed as it may lead to fatal hemorrhage. It is important to note that failure to locate a bleeding source with upper endoscopy does not eliminate aortic graft enteric fistula from the differential as a fistula can occur at any location throughout the GI tract.
Contrast-enhanced CT scan is the mainstay of current radiographic imaging modalities to assist with identification of graft enteric fistula. Common findings include periaortic gas/fluid, anastomotic aneurysm, bowel wall thickening, and other inflammatory changes. Rarely, enteric extravasation of intravenous contrast will be identified.
Catheter-directed arteriography may provide clues, such as anastomotic aneurysm/pseudoaneurysm; however, it provides limited information of graft infection and would only be able to identify a fistula tract if bleeding was evident at a rate of greater than 0.5 mL/minute. It is useful, though, in the preoperative setting to define anatomy in preparation for arterial reconstruction.
Tagged leukocyte (indium-111) scanning is fairly sensitive in detecting aortic graft infection; however, it is ineffective in detecting a graft enteric fistula. Nuclear imaging functions best as an adjunct to helical CT imaging. Clinical suspicion along with a positive scan should prompt operative intervention.
Diagnosis and Treatment
Based upon the data available, the leading diagnosis of the above patent is aortic graft enteric fistula.
Nonoperative management is rarely successful in the treatment of aortic graft enteric fistula and is only used in a palliative setting if the patient is unfit for repair. Therapy is centered around repair of fistula and eradication of infection. Broad-spectrum antibiotics are initiated preoperatively. CT imaging is often adequate for operative planning, but assessment of upper extremity perfusion is also important to assess inflow sites if axillofemoral graft is planned. In the hemodynamically unstable patient, control of hemorrhage is the primary goal. Conventionally, this is performed through a midline laparotomy or alternatively through a retroperitoneal approach. Supraceliac clamping may be required for initial control of bleeding until further dissection is carried out. An alternative to a supraceliac clamp would be balloon occlusion through the existing graft up to the same level. Following vascular control, graft excision along with bowel repair and arterial reconstruction is performed. Due to the nature of an emergency operation, patient morbidity and mortality may be increased due to several factors including preoperative hypotension, necessity of supraceliac clamping for control, and longer-than-usual aortic clamp times. An additional application in the emergency setting of a graft enteric fistula is the use of endovascular stents for fistula coverage. Currently, this option should not be viewed as curative, as the fistulous connection will not be absolved and the endograft will likely become seeded with enteric bacteria. It can, though, be utilized as a temporizing measure prior to a formal repair or a palliative treatment in a terminal patient. This maneuver will allow time for nutritional optimization, antibiotic therapy, and case planning that may include arterial reconstruction prior to graft excision. It should also be considered in those patients with overall decline since their initial procedure who may no longer tolerate an open exploration.
In the stable patient, several acceptable alternatives exist for graft enteric fistula repair. Extra-anatomic bypass has been the mainstay of reconstruction in past years. This repair can be either staged with infected graft excision occurring in the following 0 to 3 days or sequential with excision occurring in the same setting. Traditional configuration is an axillary-femoral-femoral bypass with additional outflow options of superficial femoral artery or profunda femoris in the setting of groin involvement. The distal descending thoracic aorta is an alternative inflow source, which would be tunneled in an uninvolved retroperitoneal plane.
Autogenous tissue in the form of femoral veins has proven in recent years to be a suitable option for reconstruction of a neoaortoiliac system (NAIS). This approach is routinely performed in the same setting of graft excision and can be fairly time-consuming; however, it can be staged with vein exposure left in situ followed by explant and reconstruction in the following day. Construction of NAIS does avoid the decreased long-term patency of certain extra-anatomic configurations and the complications associated with an aortic stump failure. Similarly, cryopreserved homograft is an available option that allows for in-line aortoiliac reconstruction while eliminating the morbidity associated with a femoral vein harvest. Criticisms of this technique include excessive cost, lack of immediate availability, advanced conduit calcification, and aneurysm degeneration.
An additional option for arterial reconstruction is in the form of rifampin-impregnated Dacron graft. This is an inexpensive option with a fairly high patency rate that is readily available in most institutions. Graft should be soaked in 600-mg rifampin diluted in a saline solution for more than 30 minutes prior to implantation. When used with an omental wrap and long-term antibiotics in patients without excessive perigraft purulence, rifampin-soaked Dacron is a practical option for reconstruction (Fig. 2).
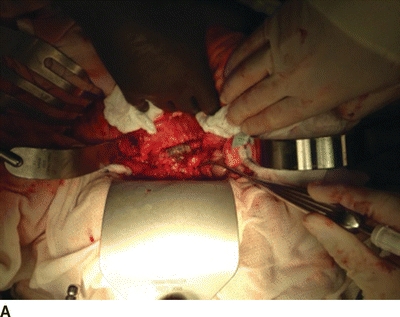
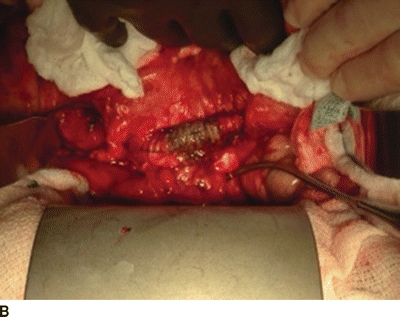
FIGURE 2 Bile staining of Dacron graft at site of aortic graft enteric fistula. A: Shows entire operative filed. B: Demonstrates magnified view of bile stained graft.



