Chapter 138
Aortic Dissection
Mark F. Conrad, Richard P. Cambria
Acute aortic dissection is the most common catastrophic event affecting the aorta, with an incidence exceeding that of ruptured abdominal aortic aneurysm. The first report of aortic dissection and the concept of a true and false lumen is attributed to Shekelton1 in the early 1800s. The term anurysme dissequant, or dissecting aneurysm, introduced by Laennec in 1819,2 remains a source of confusion because acute dissections can occur in both dilated diseased aortas and aortas of normal diameter in seemingly healthy individuals. The terms dissection and aneurysm should not be used interchangeably; although dissection can occur in a preexistent degenerative aneurysm and aneurysms can complicate chronic dissections, the presence of one does not depend on the other. An additional source of diagnostic confusion is the presence of other pathologies of the thoracic aorta, such as intramural hematoma and penetrating aortic ulcer, which have clinical and radiographic similarity with acute dissection.
Aortic dissection is a lethal disease. Early studies indicated that without treatment, the majority of patients with the condition died within 3 months of presentation and few survived the chronic phase more than 5 years because of aneurysmal degeneration and rupture of the outer wall of the false lumen.3,4 Despite improvements in both medical and surgical therapeutic options, overall mortality associated with acute dissection remains significant. In a population-based epidemiologic study by Clouse et al,5 38% of aortic dissections were diagnosed at autopsy. This substantial mortality rate in undiagnosed patients underscores the importance of early diagnosis and initiation of appropriate therapy. Indeed, Khan et al6 predicted that the mortality of acute dissection that is left untreated will exceed 22.7% within 6 hours, 50% within 24 hours, and 68% within the first week. Death due to an acute dissection of the ascending aorta is usually secondary to the central cardioaortic complications of aortic rupture into the pericardium, acute aortic regurgitation, and coronary ostia compromise,7,8 whereas descending aortic dissections are more commonly associated with death from end-organ compromise due to obstruction of visceral or extremity vessels.9,10
The International Registry of Acute Aortic Dissection (IRAD) is a multinational registry that began enrolling patients in January of 1996. It initially included 12 centers in 6 countries and has grown to 24 referral centers in 11 countries. All patients with acute aortic dissection confirmed by diagnostic imaging studies, by direct visualization in the operating room, or at autopsy are included in the registry. Data are entered from a prospectively collected questionnaire of 290 variables and have been externally validated at each site.11 This project has provided contemporary insight into the short-term and now long-term outcomes of acute dissection and proposed therapeutic options and is referenced often herein.12,13
In this chapter, we review the classification, pathologic anatomy, clinical presentation, and diagnostic and treatment modalities for acute aortic dissection with emphasis on the role of the vascular/endovascular surgeon. Although a thorough understanding of all components of acute dissection seems requisite for those who propose to treat aortic dissection, the technical principles of graft replacement of the ascending aorta, being the province of cardiac surgeons, is not detailed. Open graft replacement for treatment of descending aortic dissection is rarely indicated, so it is anticipated that the vascular/endovascular surgeon will become the primary interventionalist in the care of patients with acute dissection of the descending aorta and the complications of peripheral vascular compromise. Finally, treatment of the principal late complication of acute aortic dissection—the development of thoracoabdominal aneurysms—is considered in Chapters 134-137.
Classification
Aortic dissections are classified according to the anatomic location of the entry tear and the time between onset of symptoms and patient presentation.
Temporal
A dissection is considered acute for the first 2 weeks after the initial onset of symptoms and subsequently becomes chronic. Although such a designation appears arbitrary, it is based on autopsy studies showing that 74% of patients who die from aortic dissections do so in the first 14 days.14 Accordingly, such temporal classification combined with the anatomic location of the entry tear (see later) can have immediate therapeutic implications.
Anatomic
Anatomically, aortic dissection is classified on the basis of the location of the intimal tear and the extent of the dissection along the aorta. Two classification schemes are used to describe aortic dissections. The original, proposed by DeBakey et al15 in 1965, delineates both the origin of the entry tear and the extent of the descending aortic dissection. The scheme (Fig. 138-1) uses the following classification:
Type II: Dissection originates in and is confined to the ascending aorta.
Type IIIa: Dissection originates in the descending aorta and is limited to same.
Type IIIb: Dissection involves the descending aorta and variable extents of the abdominal aorta.
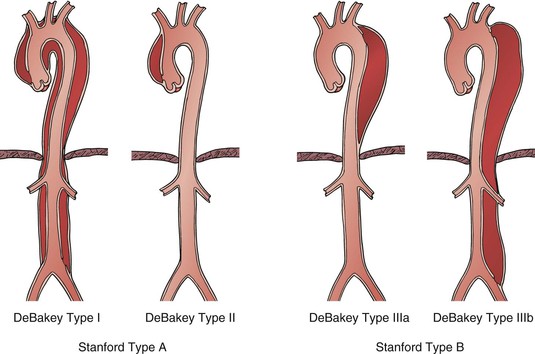
Figure 138-1 Acute aortic dissection can be classified by the original DeBakey system, based on location of proximal tear and distal extent of the dissection flap, or the Stanford system that uses location of proximal tear alone.
The Stanford classification, described by Daily et al16 in 1970, simplified the anatomic classification based on the origin of the entry tear alone. A Stanford type A dissection originates in the ascending aorta and therefore encompasses DeBakey type I and II dissections, whereas a Stanford type B dissection originates in the descending aorta distal to the origin of the left subclavian artery (DeBakey types IIIa and IIIb; see Fig. 138-1). Because the origin of the entry tear is the key predictor of early outcomes, most patients are now classified as having either Stanford type A or B dissection at the time of presentation in order to direct initial therapy. Prompt graft replacement of the ascending aorta is the appropriate treatment for the majority (for exceptions, see later) of patients with Stanford type A dissections because these are associated with a high risk of lethal cardioaortic complications (principally, aortic rupture and myocardial ischemia from extension into the coronary arteries) in the hours and days after symptom onset. Alternatively, patients with Stanford type B dissections are managed initially with medical therapy unless one or more complications reviewed later develops.
Epidemiology
Population-based studies have estimated the incidence of acute aortic dissection to range from 2.9 to 3.5 per 100,000 person-years.5,17 Factors that predispose one to development of aortic dissection include age, hypertension, and structural abnormalities of the aortic wall.6,11 Men are more frequently affected with a male-to-female ratio of 4 : 1 reported in one IRAD series.18 In our cumulative experience, type A dissections account for 60% of cases, and this figure is consistent with the IRAD data wherein 62.5% of 1417 patients presented with a type A dissection.12 The incidence of type A dissection peaks between 50 and 60 years, whereas type B dissections occur more frequently between 60 and 70 years of age.11 Hypertension is the rule and was present in 70% of patients in the IRAD database (Table 138-1).18
Risk Factors for Dissection
Cardiovascular Conditions
Cardiovascular conditions, such as acute myocardial infarction and sudden death, have been shown to demonstrate certain chronobiologic patterns of occurrence,19,20 and the same is true of acute aortic dissection.21 The onset of dissection occurs most frequently in the morning hours, between 6 AM and 12 PM, and more often in the winter (28%) than the summer (20%) independent of endogenous climate.21,22 Aortic wall structural abnormalities and the presence of a bicuspid aortic valve with or without its accompanying aortic root dilatation are well-established risk factors for ascending aortic dissections. Indeed, the presence of a bicuspid aortic valve has been documented in 7% to 14% of all aortic dissections.11,23 Other aortic diseases, such as coarctation of the aorta, annuloaortic ectasia, chromosomal abnormalities (Turner’s and Noonan’s syndromes), aortic arch hypoplasia, and hereditary conditions (Marfan’s and Ehlers-Danlos syndromes), are also risk factors for the development of acute aortic dissection.24 Marfan’s syndrome accounts for 50% of cases of acute aortic dissection in patients younger than 40 years of age (see Chapter 143).18
Pregnancy
It was previously reported that in women younger than 40 years, 50% of aortic dissections occur during pregnancy25; however, a more recent review of the IRAD data showed an incidence of 13%.18 Preeclampsia with resultant hypertension is the most common etiology of peripartum aortic dissection, but pregnant women with Marfan’s syndrome are also at high risk; the presence of a dilated aortic root (>4 cm) is the best predictor of dissection in the pregnant patient with Marfan’s syndrome.26 The most common site of aortic dissection in young (<40 years) patients is the ascending aorta involving the sinuses of Valsalva or the sinotubular junction, whereas type A dissections in older patients are more likely to originate in the ascending aorta.18
Cocaine Abuse
Cocaine ingestion, a rare cause of acute aortic dissection in otherwise healthy individuals, has an incidence as high as 37% in an urban setting but was present in less than 1% of the IRAD population.18,27,28 Daniel et al,28 reviewing the characteristics of 16 patients who had used cocaine within 24 hours of onset of acute aortic dissection, found that the prototypical patient was a young (average age 47 years), male (75%) smoker (100%) with a history of hypertension (70%) and no particular trend toward dissection type.28 One possible mechanism is related to catecholamine release, which causes a triad of profound hypertension, vasoconstriction, and increased cardiac output. This creates a dramatic, acute increase in ventricular contraction (shearing) force (dP/dt) on the aortic wall. The intimal tear occurs most often at the ligamentum arteriosum, where the aorta is relatively fixed and unable to tolerate the accelerating aortic pressure wave generated by the profound tachycardia and malignant hypertension.28,29
Pathologic Anatomy of Acute Aortic Dissection
Intimal Tear
The process of aortic dissection is dynamic and can occur anywhere along the course of the aorta, resulting in a wide spectrum of clinical manifestations. The pathognomonic lesion is one of an intimal tear followed by blood surging either antegrade (typically) or retrograde (depending on the hemodynamic gradient between the true and false lumina) and cleaving the intima and media layers of the aortic wall longitudinally for a variable distance.6,30 The typical tear is transverse, not circumferential, and the intimomedial layer can be cleaved both longitudinally and circumferentially.6 The adventitially bound blood-filled space created between the dissected layers of the aortic wall becomes the false lumen. Fenestrations (connections between the true and false lumina) occur within the intimal flap downstream, usually at branch vessel ostia, which are cleaved by the dissection process. These serve as sites of reentry of blood flow into the true lumen, thus maintaining false lumen patency. The presence of an “intimal flap,” representing the intimomedial septum between true and false lumina, is the most characteristic pathology in acute aortic dissection. The origin of the intimal flap/tear is in the ascending aorta in 65%, the descending aorta in 25%, and in the arch and abdominal aorta in 10% of patients.11 In the descending aorta, the intimal tear typically originates within a few centimeters of the left subclavian artery because this segment of the aorta is subject to the greatest dP/dt pressure fluctuations.14,31,32 In the usual pattern of a Stanford type B dissection, the cleavage plane progresses with the distal false lumen on the left posterolateral aspect of the aorta (80% of patients); the celiac, superior mesenteric, and right renal arteries typically emanate from the true lumen, and the left renal artery arises from the false lumen.14 We must emphasize, however, that variations in this pattern are frequently encountered.
Cystic Medial Necrosis
One pathologic process that is associated with increased risk of aortic dissection is medial degeneration of the aortic wall (cystic medial necrosis), which diminishes the structural integrity of the aortic layers.32,33 The central lesion appears to be deterioration of medial collagen and elastin fibers through elastolysis; this is considered a factor in most cases of aortic dissection.34 In particular, classic cystic medial necrosis appears to be an essential feature of several hereditary conditions, such as Ehlers-Danlos syndrome and Marfan’s syndrome.24 Yet specific connective tissue diseases account for only 10% to 15% of all acute aortic dissections.11 Even in “normals,” in whom dissections occur without any antecedent diagnosis of syndromic diseases, the degree of medial degeneration still tends to be greater than expected as part of normal aging. The exact cause of this medial degeneration remains unclear, but advanced age and hypertension appear to be the most important factors.21,34,35
Atherosclerosis
Atherosclerosis has not been considered an important etiologic feature of acute aortic dissection and was present in only 31% of patients in the IRAD.36 However, Jex et al37 noted either gross or microscopic atheroma in 83% of patients in their series. Atherosclerotic plaque may, in fact, be protective in serving to terminate the dissection process because the transmural inflammatory nature of atherosclerosis may serve to fuse the aortic layers.38 The presence of an atherosclerotic aneurysm manifesting with concurrent aortic dissection is uncommon, occurring in only 14% to 15% of patients in later series.36,39 The unusual coexistence of an aortic dissection that originates in and/or involves a preexistent atherosclerotic aneurysm appears to change the natural history, and rupture is the likely scenario. In a review of 325 patients with aortic dissection, rupture in the abdomen occurred only in the setting of antecedent degenerative, atherosclerotic aneurysm.38 These findings support the posture of treating such type B dissections as “complicated,” and initial surgical priority should be given to the aorta in which both entities are present (usually the infrarenal abdominal aorta).
Pathogenesis of Malperfusion Syndromes
Malperfusion syndrome occurs when there is end-organ ischemia secondary to aortic branch compromise from the dissecting process. It can involve one or more vascular beds simultaneously, with early symptoms often subtle and of varying severity over the hours and days after initial symptom onset. The terms aortic branch compromise and malperfusion syndrome should not be used interchangeably because obstruction is often subtotal, producing variable degrees of end-organ ischemia and certain affected vessels (e.g., subclavian and celiac arteries) may not produce critical ischemia, even with total occlusion, owing to the presence of collateral circulation. Consistently in several series, aortic branch compromise is present in up to 31% of patients with acute aortic dissection,10,36,40,41 and progression to the malperfusion syndrome correlates with early mortality.9,10,36 Virtually any aortic branch can be affected, and as intuitively suspected, the morbid clinical events vary as a function of the vascular territory involved. Mesenteric involvement is associated with intestinal infarction, whereas subclavian and/or lower extremity occlusive events are often well tolerated (Fig. 138-2).
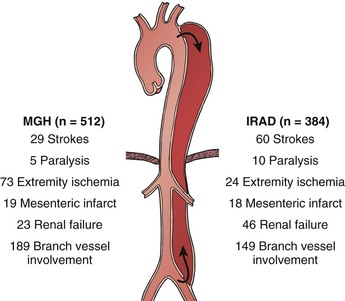
Figure 138-2 Comparison of Massachusetts General Hospital (MGH) and International Registry of Acute Aortic Dissection (IRAD) experiences with peripheral vascular complications by after acute aortic dissection. Differences between numbers of branch vessels involved and clinical events represent asymptomatic lesions.
Mechanism
Identifying the mechanisms of branch compromise is a critical step in formulating effective treatment plans. The anatomic and physiologic variables underpinning any compromised vascular bed are as follows: (1) the percentage of aortic circumference dissected, (2) the presence of a distal reentrant focus in the false lumen or true lumen outflow, (3) and the topography of branch ostia in relation to the true versus false lumen.7 In the minutes after an aortic dissection is initiated, the true lumen (representing the remnant of the original aortic lumen) collapses to a variable degree and the false lumen expands.42 The adventitially bound outer wall of the false lumen must expand to a larger diameter in order to accommodate the same wall tension at a given blood pressure, as governed by the law of LaPlace. In contrast, the true lumen, which contains the majority of the elastic components of the aortic wall, undergoes radial elastic collapse.42 Therefore, the extent to which the true lumen recoils and the false lumen expands (i.e., their respective cross-sectional areas) depends on the percentage of the total aortic circumference involved with the dissection. In the presence of a deep proximal tear and the absence of distal fenestrations, the mean false lumen pressure increases, leading to compression of the true lumen43 (Fig. 138-3). Compression of the true lumen leads to impaired perfusion of distal structures and should increase the index of suspicion for visceral and renal ischemia. Finally, the topographic relationship of the true and false lumina and the potential for extension of the dissection into the aortic branch itself are the anatomic factors that determine the mechanism of malperfusion syndrome (see Fig. 138-3).
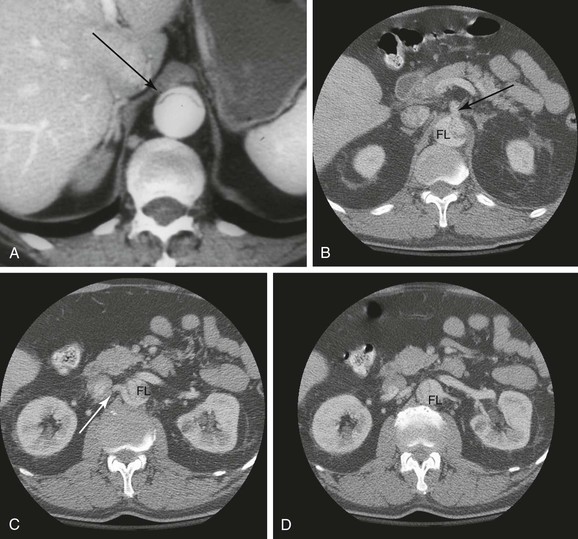
Figure 138-3 CT angiographic findings in acute aortic dissection. A, Axial image of aortic dissection with compressed true lumen (arrow). B, Axial image of aortic dissection with superior mesenteric artery arising from the true lumen (arrow). FL, False lumen. C, Axial image of aortic dissection with right renal artery (arrow) arising from the compressed true lumen. D, Axial image of aortic dissection with left renal artery originating from the false lumen.
Two mechanisms for aortic branch vessel compromise have been identified, each of which has specific treatment implications in the management of malperfusion syndromes as originally described by Williams et al.42
Dynamic Obstruction
In dynamic obstruction, the compressed true lumen is unable to provide adequate volume flow, or the dissection flap may prolapse into the vessel ostium, which remains anatomically intact (Fig. 138-4A). This more common mechanism of branch compromise is responsible at least in part for some 80% of malperfusion syndromes.44 The severity of the true lumen collapse and the degree of aortic-level ostial vessel occlusion is determined by the circumference of the dissected aorta, cardiac output, blood pressure, heart rate, and the peripheral resistance of the outflow vessel.45 Pulse deficits based on dynamic obstruction may wax and wane over time owing to variability of the aforementioned factors.7,46 Chung et al45 modeled the anatomy and physiologic conditions of a Stanford type B aortic dissection in vitro to study the hydrodynamic effects that worsen true lumen collapse. In conditions of equal outflow from each lumen in pulsatile flow, increasing the size of the aortic entry tear from 10 mm to 30 mm (i.e., increasing the amount of aortic circumference involved) significantly aggravated the extent of true lumen collapse. On the basis of these observations, they concluded that the movement of the dissection flap to produce dynamic obstruction of any branch vessel is related to the size of the entry tear, the limitation of false lumen outflow, and increased true lumen outflow produced by falling peripheral resistance.
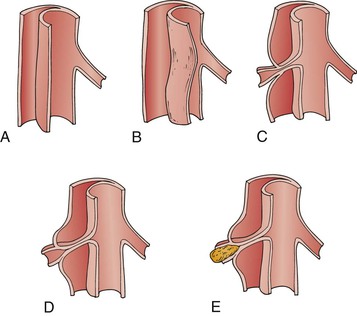
Figure 138-4 Mechanisms of aortic branch obstruction in acute dissection. A and B, In dynamic obstruction, the septum may prolapse into the vessel ostium during the cardiac cycle, and the compressed true lumen flow is inadequate to perfuse branch vessel ostia, which remain anatomically intact. C to E, Near-complete circumferential dissection with static obstruction—the cleavage plane of the dissection extends into the ostium and compromises inflow. Thrombosis beyond the compromised ostia may further worsen perfusion.
Static Obstruction
In acute dissection, the false lumen is highly thrombogenic as a result of the exposed adventitial and medial layers. Thrombus formation may occur in the blind end of the dissection column; more often, “reentrant” foci maintain false lumen flow.47 If the blind end or the propagating end of the dissection column enters and constricts the ostia of a branch vessel, organ injury can occur by thrombosis or hypoperfusion of the involved vessel. This mechanism for malperfusion syndrome involves extension of the dissecting process into the branch vessel proper, narrowing it to a variable degree—and has been termed static obstruction.44 This obstruction is unlikely to resolve with restoration of aortic true lumen flow alone, and some manipulation of the vessel itself (e.g., stent, bypass graft) is typically required. Alternatively, the more common scenario occurs when the dissection process shears the aortic intimomedia around the vessel ostium and the vessel anatomy itself remains intact with flow provided by the false lumen. Therefore, most false lumen branches rarely show evidence of ongoing malperfusion47 (Fig. 138-4B).
Clinical Presentation
Pain
The most common presenting symptom of acute aortic dissection is pain (located in the back, abdomen, or chest), reported by more than 93% of patients, with 85% specifying an abrupt onset.11,48 The pain is typically described as anterior in location in type A dissections but is more often experienced in the back in type B dissections (78% vs. 64%, respectively).11,23 Although the classic description of pain associated with aortic dissection is ripping or tearing (50%), patients more frequently complain of sharp, stabbing pain (68%) and less often experience migratory symptoms (19%).11 Pain has been localized to the abdomen in up to 21% of patients with type A and 43% of patients with type B dissections.11 In such patients, a high index of suspicion for mesenteric vascular compromise is warranted. Typically, the pain is severe, causing the patient to seek medical attention within minutes to hours of onset, and has been described as “the worst ever” by nearly 90% of patients.11 The control of pain with antihypertensive therapy is a mainstay of the early management of acute aortic dissection, and the recurrence of pain implies failure of medical therapy, warranting further imaging to direct therapy. However, in the absence of clinical or radiographic signs of pathoanatomic changes, medical therapy remains appropriate management of patients with early recurrent pain after type B dissection.49
Syncope
Syncope may complicate the presentation of acute aortic dissection in 5% to 10% of patients, and its presence often indicates the development of cardiac tamponade or involvement of the brachiocephalic vessels.50 Overall, patients in the IRAD who presented with syncope were more likely to have a type A dissection than type B (19% vs. 3%, P < .001) and to have cardiac tamponade (28% vs. 8%, P < .001).51 Similarly, they were more likely to have a stroke (18% vs. 4%, P < . 001) and to die in the hospital (34% vs. 23%, P = .01). Patients presenting with syncope had a higher rate of severe complications (tamponade, stroke, death), but in almost half, the complications were not the cause of the loss of consciousness. The mechanisms underlying this fact may be related to other pathophysiologic perturbations, such as vasovagal events and direct stretching of baroreceptors in the aortic wall.52,53
Neurologic Symptoms
Spinal cord ischemia from the interruption of intercostals vessels is clearly more common with type B aortic dissections, occurring in 2% to 10% of all patients.54 Direct compression of any peripheral nerve can occur rarely, resulting in paresthesia (lumbar plexopathy), hoarseness of voice (compression of recurrent laryngeal nerve), or Horner’s syndrome (compression of sympathetic ganglion).55–57
Hypertension
On initial physical examination, hypertension is present in 70% of type B dissections but only 25% to 35% of type A dissections. The presence of hypotension complicating a type B dissection is rare (<5% of patients) but may be present in 25% of dissections that involve the ascending aorta, potentially as a result of aortic valve disruption or cardiac tamponade.11 The malperfusion of brachiocephalic vessels by the dissection may falsely depress brachial cuff pressures.58 Hypertension that is refractory to medical management is common in type B dissections, occurring in 64% patients.59 However, because this refractory hypertension is usually not associated with renal artery compromise or aortic dilatation, continued medical management is warranted.
Peripheral Vascular Complications
Peripheral vascular complications are common, occurring in 30% to 50% of patients in whom the aortic arch and/or the thoracoabdominal aorta is involved.10,40,41 Cambria et al10 were the first to quantify the contribution of said complications to overall mortality. They noted that in patients with peripheral vascular complications not involving the ascending aorta, the brachiocephalic trunk was involved in 14% of patients, the common carotid arteries in 21%, the left subclavian artery in 14%, and the ileofemoral arteries in 35%.10 This distribution was again borne out by the IRAD data 15 years later.60 Lauterbach et al9 found that patients presenting with pulse deficits more often have neurologic deficits, coma, and hypotension, and a deficit of the carotid artery pulse strongly correlates with fatal stroke.9 In this population, mortality is clearly linked to the number of pulse deficits at the time of presentation. Indeed, IRAD data showed that within the first 24 hours, 9.4% of patients with no deficits died, 15.8% of patients with one or two deficits died, and 35.3% of patients with three or more deficits died.60 Not surprisingly, it is uncommon for isolated lower extremity pulse deficits to cause death as a result of lower extremity ischemia or its sequelae.10 Despite this seemingly favorable prognosis, leg ischemia caused by acute aortic dissection remains a marker of extensive dissection and may be accompanied by compromise of other vascular territories, and the clinical course of lower extremity ischemia can be quite variable because up to one third of patients affected may demonstrate spontaneous return of pulses.10
Diagnostic Evaluation
In the United States, acute chest pain is the chief complaint in 8.2% of all emergency department visits. This translates to almost 4.6 million patients annually.61 The majority of patients with this complaint do not have an aortic dissection, and it would be inefficient, unrealistic, and costly to obtain axial imaging in all patients with acute chest pain. Indeed, the indiscriminate application of thoracic imaging to patients with low pre-test probability of having an aortic dissection has been predicted to yield an 85% false-positive rate.62 However, aortic dissection often affects younger patients (in their 50s) and thus is not readily apparent. Indeed, physicians correctly suspect the entity in only 15% to 43% of presentations, and aortic dissection is often identified as an incidental finding during evaluation of another pathologic process.17,63 Historically, retrograde aortography was considered the reference standard in the evaluation of acute aortic dissection. However, the improvement of other techniques, such as contrast computed tomographic angiography (CTA), transesophageal echocardiography (TEE), and magnetic resonance imaging (MRI), has shifted the role of aortography from diagnostic to adjunctive during endovascular management. An evaluation of the IRAD data showed that worldwide, CTA was the initial test performed in 63% of patients and that 75% underwent CTA as part of the workup.64 TEE was also routinely used (72%), although it was the first test obtained in only 32% of patients. More than two thirds of patients required two or more imaging tests, and MRI (19%) and aortography (19%) were used with the same frequency.64 Although the choice of diagnostic modality may be predicated on local availability/practice patterns, certain essentials exist. First, the diagnosis of acute aortic dissection must be confirmed or refuted. Second, information about the extent of the dissection, the potential involvement of branch vessels, and the presence of immediate life-threatening complications such as tamponade should be provided by the modality used.
Imaging
Plain Radiography
Plain chest radiography is often the first imaging study obtained during the workup of acute chest pain, but the chest radiography findings in aortic dissection are nonspecific and never diagnostic. They include widening of the cardiac or aortic silhouette, displacement of aortic calcifications, and effusions.11,23 Widening of the mediastinum is the most common finding, whereas displaced aortic calcifications are usually seen with type A dissections.11 Pleural effusions occur most frequently in patients with type B dissections and are usually secondary to an inflammatory reaction of the mediastinal pleura.65 Because these findings are not diagnostic, additional imaging is required.
Aortography
Aortography has a sensitivity of 86% to 88% and a specificity of 75% to 94% for the diagnosis of thoracic aortic dissection, but false-negative angiograms can occur when the false lumen has thrombosed.66–69 The aortographic findings considered supportive of a diagnosis of aortic dissection include distortion of the normal contrast column, flow reversal or stasis into a false channel, failure of major branches to fill, and aortic valvular regurgitation.68 Most contemporary diagnostic paradigms have deemphasized the role of aortography because it is time consuming and invasive, incurs the risks of contrast nephropathy, and is expensive. Therefore, in contemporary practice, aortography is considered unnecessary prior to surgical repair of proximal dissection.64,70 In the management of distal dissection, this modality is used as a part of a treatment (see later) rather than diagnostic modality.
Computed Tomographic Angiography
As evidenced by the IRAD data, CT was part of the diagnostic evaluation in 75% of patients.48 CT is readily available and noninvasive, and it has a reported sensitivity of 83% to 95% and specificity of 87% to 100% for the diagnosis of acute aortic dissection.71–73 The chief limitation of CT is in the ascending aorta, where the sensitivity may drop to less than 80%, but this is readily overcome by the addition of TEE.58 A dedicated protocol to image the entire aorta is usually sufficient to provide the necessary diagnostic information. A helical CT dissection protocol gives excellent aortic imaging of the true and false lumina and approximate entry tear sites, and aids in planning intervention (Fig. 138-5). In most cases, the true lumen may be localized by its continuity with an undissected segment of the aorta.74 However, in circumferential dissection of the aortic root or when imaging of the aortic arch is omitted, this rule may be difficult to apply. The presence of intraluminal thrombus is a fairly good marker of the false lumen, but in patients with a concomitant degenerative aneurysm, thrombus may be present in the true lumen.74 The finding of greatest significance is that in the descending thoracic aorta, the false lumen is larger than the true lumen in more than 90% of cases (P < .05).74
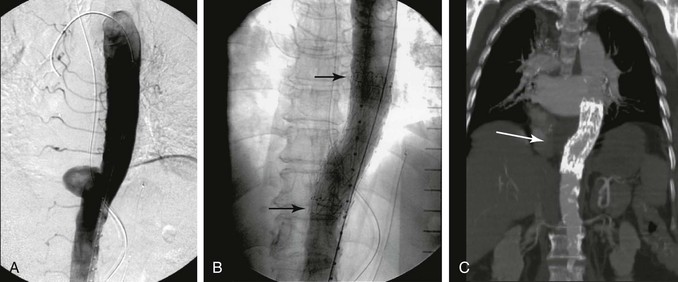
Figure 138-5 Mid descending thoracic aorta penetrating aortic ulcer (A) treated by aortic stent graft (B, procedural image, C, follow-up CTA).
The orientation and mobility of the dissection flap can be assessed with CTA and, if the dissection flap is concave toward the false lumen, a true lumen pressure deficit can be predicted with a 91% sensitivity and 72% specificity.47 The dissection flap is more often noted to be in a curved orientation (63% of cases) in the acute phase; the flap was flat in 75% of cases of chronic dissection in one series.74 As displayed in Figure 138-3, a slit-like compressed true lumen is perhaps the key radiographic finding that should substantially raise the index of suspicion for renal, visceral, or lower extremity malperfusion syndrome.
Three-dimensional CT scan reconstructions can aid treatment planning, but axial imaging affords the best opportunity to detect topographic relationships of the true and false lumina and potential aortic branch compromise. Although it is appropriate to operate on acute type A dissections on the basis of CT findings alone, the IRAD data show that most patients undergo preoperative CT (71%) and TEE (77%) (either preoperative or intraoperative) when the clinical and/or laboratory signs dictate the need for urgent revascularization.75 In comparison with other modalities, CT is the least operator dependent, provides useful anatomic correlates for surgical and endovascular therapy, and most reliably collects information for follow-up analysis and measurement.
Echocardiography
The sensitivity and specificity of transthoracic echocardiography (TTE) range from 35% to 80% and 40% to 95%, respectively.76,77 The chief technical limitations of transthoracic echocardiography, whether performed in the suprasternal or subcostal views, are the presence of narrow intercostal spaces, obesity, and emphysema. In addition, false-positive results have been reported in transthoracic echocardiography imaging of the ascending aorta due to artifacts.78 Transesophageal echocardiography (TEE) overcomes the limitations of surface echocardiography because of the anatomic proximity of the esophagus to the aorta. The sensitivity of TEE has been reported to be as high as 98%, and the specificity ranges from 63% to 96%.79–81 Advantages of TEE include wide availability, ease of use, and bedside capability. In addition, TEE possesses the ability to detect entry tear sites, false lumen flow/thrombus, involvement of the arch or coronary arteries, degrees of aortic valvular regurgitation, and pericardial effusions. The addition of color-flow Doppler imaging patterns may decrease false-positive TEE results by showing differential flow velocities in the true and false lumina.82 The chief limitations of TEE are the anatomic blind spot in the distal ascending aorta and arch secondary to the air-filled trachea and left main stem bronchus and the inability to document dissection extension beyond the diaphragm.83 Despite these shortcomings, TEE can be particularly useful in delineating dissection and relevant surgical pathology in the ascending aorta, and therefore, is chiefly applied in this territory.84 Moreover, in the unstable patient with a suspected acute dissection in the ascending aorta, TEE may be performed in the operating room to expedite diagnosis and definitive therapy.
Magnetic Resonance Imaging
Magnetic resonance imaging has an overall sensitivity and specificity for diagnosis of aortic dissection in the range of 95% to 100%.64,85,86 MRI can detect the site of the entry tear, the extent of the dissection, potential branch vessel involvement, and differential true versus false lumen flow. The overall sensitivity and specificity for diagnosis of branch vessel involvement are 90% and 96%, respectively.87 The chief limitations of MRI include lack of immediate availability, long examination times, and lack of monitoring for critically ill patients. In addition, patients who have had pacemakers, aneurysm clips, or ocular implants are not candidates for MRI.
Treatment
Optimal treatment of acute dissection is predicated on timely diagnosis and a thorough understanding of the anatomic extent of the pathology. Prompt institution of intravenous antihypertensive medications to lower systemic blood pressure and pulse (dP/dT) is a key element of initial therapy for all patients, with the goal of stabilizing the extent of the dissection, reducing intimal flap mobility, relieving dynamic aortic branch obstruction, and decreasing the risk of rupture. Mortality in the acute phase of a proximal dissection may exceed 1% per hour in relation to the central cardioaortic complications of tamponade, acute aortic valvular insufficiency, and coronary obstruction. Thus, prompt ascending aortic graft replacement with or without aortic valve repair/replacement is the treatment of choice for the majority of patients with acute type A aortic dissection. For patients with type B dissections, the catastrophic complication of rupture is uncommon, except in those patients who present with advanced false lumen dilatation or the equivalent of aneurysm formation at the aortic entry site.7 Furthermore, in stable patients with uncomplicated type B dissections, surgical therapy (i.e., graft replacement of the aortic entry tear site) has not demonstrated superiority over medical or interventional therapy.48
Aortic branch compromise by the propagating false lumen and subsequent malperfusion syndrome may complicate the initial presentation of patients with extensive type B dissections. A complication-specific approach involving open surgical and endovascular options to treat such malperfusion syndromes remains the standard of care and is reviewed here. The application of stent-graft repair at the entry tear may alter this paradigm in the near future.
Medical Treatment
Medical treatment of aortic dissection was first advocated in the 1960s by Wheat (ironically, a surgeon) et al88 as an alternative for those patients too ill to withstand surgical therapy. Currently, medical management in an intensive care unit is the initial therapy for virtually all patients with the tentative diagnosis of aortic dissection. The immediate management of acute aortic dissection is directed toward reducing the hemodynamic forces that have initiated and propagated the intimal tear and cleavage of the aortic wall. The goal of medical therapy is to reduce systolic blood pressure and dP/dT and thereby reduce the forces predisposing the dissected aorta to rupture or compromise of branch vessels.89 Intravenous antihypertensive therapy should be started in all patients in whom acute aortic dissection is suspected, with the exception of those with hypotension.89 For those patients with hypotension in the setting of acute dissection, an expeditious evaluation for tamponade is warranted, but percutaneous pericardiocentesis as a temporizing measure is not advised because it often accelerates bleeding or shock.90
In contemporary practice, a combination of a beta blocker and a vasodilator is standard medical therapy. In addition, the beta blocker should be initiated before the direct vasodilator (i.e., sodium nitroprusside); otherwise, reflex sympathetic stimulation from direct vasodilatation will stimulate cathecholamine release and resultant increases in dP/dT, opposite of the desired effect. The cornerstone of medical therapy is the reduction of both dP/dT and arterial blood pressure. For the acute reduction of dP/dT, an intravenous beta blocker is infused in incremental doses until evidence of effective beta-blockade is achieved, usually indicated by a heart rate of 60 to 80 beats per minute. Beta blockers that achieve both α- and β-adrenergic blockade (such as labetalol) may achieve both dP/dT reduction and blood pressure lowering. Short-acting beta blockers (like esmolol) may be particularly useful as a test of beta-blockade tolerance in patients at risk for bronchospasm or chronic obstructive pulmonary disease (COPD) flare. In these patients, a cardioselective betablocker, such as atenolol or metoprolol, may be desirable. For the acute reduction of arterial pressure, the direct vasodilator sodium nitroprusside is very effective and should be used after beta blockade is achieved.
Patients should be admitted to the intensive care unit during the acute period, with continuous blood pressure monitoring via an intra-arterial catheter, telemetry monitoring of cardiac rhythms, and hemodynamic surveillance involving a Foley catheter and pulmonary artery catheter if necessary. Once blood pressure has been controlled to a systolic of 105 to 120 mmHg (or mean of 60-70 mmHg) and the pain has resolved, the patient can be transitioned to oral antihypertensives. Patients who are managed medically should be monitored with serial surveillance imaging studies that should consist of contrast CT angiograms, the first should performed prior to discharge from the initial hospitalization and subsequent images at 6-month intervals. Once a dissection has been stable for two scans, follow-up imaging can be obtained on a yearly basis.91
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree