Current recommendations for the antithrombotic management of patients receiving oral anticoagulation (OAC) who undergo percutaneous coronary intervention with stent implantation (PCI-S) are based on limited and relatively weak data. To broaden and strengthen available evidence, the management and 1-year outcomes of OAC patients who underwent PCI-S and were included in a prospective, multicenter registry from 2003 to 2007 were evaluated. Among the 632 patients receiving OAC, mostly because of atrial fibrillation (58%), who underwent PCI-S, mostly because of acute coronary syndromes (63%), dual-antiplatelet therapy with aspirin and clopidogrel was the most frequently prescribed at discharge (48%), followed by triple therapy with OAC, aspirin, and clopidogrel (32%) and OAC plus aspirin (18%). The choice of antithrombotic therapy largely matched the thromboembolic risk profiles of patients, with the prescription of regimens including OAC predicted by the presence of non-low-risk features. The cumulative 1-year occurrence of major adverse cardiovascular events was as high as 27% and was not significantly different among the 3 treatment groups. Stroke and stent thrombosis were limited to 2% and 3%, respectively, and although no significant differences were found among the 3 groups, stroke was 4 times less frequent when OAC, with either 1 or 2 antiplatelet agents, was administered. Major bleeding was also limited to 3%, with no significant differences among the 3 groups. In conclusion, these findings suggest overall real-world management of OAC patients who undergo PCI-S that is in accordance with their clinical risk profiles and give further support to the reported efficacy and safety of triple therapy for the optimal treatment of these patients.
The antithrombotic management of patients committed to long-term oral anticoagulation (OAC), because of atrial fibrillation at moderate to high thromboembolic (TE) risk, mechanical heart valve implantation, recent venous thromboembolism, or history of cardiac embolism, who undergo percutaneous coronary intervention with stent implantation (PCI-S) is cumbersome, because of the need to effectively prevent TE events, recurrent ischemia, and stent thrombosis, while minimizing the risk for bleeding complications. On the basis of recent consensus reports and official guidelines, triple therapy (TT) with vitamin K antagonists, aspirin, and clopidogrel is the recommended antithrombotic regimen for these patients, because of its highest efficacy in preventing TE complications compared to dual-antiplatelet therapy (DAPT) with aspirin and clopidogrel or the combination of OAC and a single antiplatelet agent. This result, however, comes at the price of an increased incidence of major bleeding, apparently increasing with the duration of TT. Although these findings have been confirmed by 2 recent meta-analyses, it must be acknowledged that the overall evidence on which they are based is weak. Nearly all the studies, in fact, were small, retrospective, and single center, except for a few in which patients either were included at several centers or were enrolled in large-scale, multicenter registries, in which, however, the analysis of the OAC population was performed post hoc. More recent, larger scale studies have raised further questions regarding the true efficacy and safety profile of TT by reporting a significant increase only in minor, rather than major, bleeding in the absence of significant differences in the occurrence of thromboembolism and/or cardiac events. Because of this persistent uncertainty, we aimed to evaluating the antithrombotic management and 1-year clinical outcomes of patients receiving OAC who underwent PCI-S and were enrolled in the large multicenter prospective Registro Angioplastiche Emilia-Romagna (REAL).
Methods
REAL is a large, dynamic, multicenter, regional registry of percutaneous coronary intervention, in which the clinical and procedural data of all consecutive patients who undergo percutaneous coronary intervention at 13 hospitals in the Italian region of Emilia-Romagna are continuously conveyed through an on-line database. REAL is based on current practice at each institution, and only written informed consent to percutaneous coronary intervention and continuous data collection are requested for inclusion. The study conformed to the principles outlined in the Declaration of Helsinki, and written informed consent was obtained from all participants.
All patients receiving OAC who underwent PCI-S from 2003 to 2007 were identified, and the individual hospital records reviewed. Baseline characteristics, including indication for OAC and PCI-S, and antithrombotic treatment at discharge were evaluated. The combined incidence at 1-year follow-up of major adverse cardiovascular events (MACVEs), including cardiovascular mortality, myocardial infarction, target vessel revascularization, stent thrombosis, stroke, and venous thromboembolism, as well as of major bleeding, were also evaluated, in the overall population and according to the antithrombotic regimen prescribed at discharge.
Follow-up data were obtained directly from the Emilia-Romagna Health Care Agency through analysis of hospital discharge records and municipal civil registries. This procedure ensured a full follow-up to 100% of patients living in the region. All repeat PCI-S procedures during follow-up were prospectively collected from each institution and matched with administrative data to address possible inconsistencies. Hospital records were reviewed for additional information whenever deemed necessary.
All deaths and causes of death were collected through the civil mortality registries. Myocardial infarction, stroke, and venous thromboembolism (i.e., deep vein thrombosis and/or pulmonary embolism) were diagnosed by local cardiologists at the hospital of admission according to standard criteria and identified through the hospital discharge records. Target vessel revascularization was defined as any reintervention (surgical or percutaneous) to treat a stenosis occurring in the same coronary vessel treated at the index procedure, within and beyond the target lesion limits. Stent thrombosis was defined according to the Academic Research Consortium criteria. Only definite thrombosis, which was identified directly from the REAL database and double checked through the analysis of hospital discharge records, was evaluated. We also assessed the incidence of major bleeding events, which were classified as intracranial or gastrointestinal and/or requiring blood transfusion or surgical intervention and/or hospitalization.
Continuous variables are expressed as mean ± SD and categorical variables as frequencies and percentages. Chi-square tests were used to compare noncontinuous variables, and Student’s t tests were used for continuous variables. The unadjusted cumulative incidence of adverse events was estimated using the Kaplan-Meier method. Univariate Cox regression analysis was used to identify independent predictors of MACVEs at 1 year. Explanatory variables associated with outcomes in univariate analysis were selected for multivariate analysis in a Cox proportional-hazards model or in a logistic regression, using a stepwise elimination procedure. Results are expressed as hazard ratios (HR) or odds ratios (OR), as appropriate, with 95% confidence intervals (CIs). Two-sided p values <0.05 were considered statistically significant. All calculations were performed using SAS version 8.2 (SAS Institute Inc., Cary, North Carolina).
Results
From 2003 to 2007, 632 consecutive patients receiving OAC underwent PCI-S in the Emilia-Romagna region and were investigated. Baseline characteristics of study participants are listed in Table 1 . The prevalence of high-risk features for cardiovascular events, such as diabetes, history of myocardial infarction, and previous percutaneous or surgical coronary revascularization, was substantial. The indication for PCI-S was mostly acute coronary syndromes, while atrial fibrillation was the most frequent indication for OAC. Non-low-risk (i.e., moderate-risk or high-risk) features for thromboembolism, defined as the presence of a mechanical heart valve, atrial fibrillation with CHADS 2 (congestive heart failure, hypertension, age ≥75 years, diabetes, and previous stroke) score ≥2, history of cardiac embolism, left ventricular thrombus, and recent (<6 months) venous thromboembolism, were present in 392 patients (62%). In the remaining 240 patients (38%), low–TE risk features, defined as the presence of atrial fibrillation with CHADS 2 score <2, biologic heart valve, remote (>6 months) venous thromboembolism, dilated cardiomyopathy, left ventricular aneurysm, and chronic ischemic heart disease, were present. In most cases, bare-metal stents were implanted.
| Variable | Value |
|---|---|
| Age (years) | 73.1 ± 8.4 |
| Men | 460 (73%) |
| Diabetes mellitus | 193 (31%) |
| History of hypercholesterolemia | 349 (59%) |
| History of hypertension | 540 (88%) |
| Active smoking | 60 (11%) |
| Family history of coronary artery disease | 134 (27%) |
| Heart failure | 221 (35%) |
| Chronic kidney disease | 55 (9%) |
| Previous myocardial infarction | 234 (37%) |
| Previous percutaneous coronary intervention | 70 (11%) |
| Previous coronary bypass | 108 (17%) |
| Previous stroke | 39 (6%) |
| Previous transient ischemic attack | 34 (5%) |
| Shock | 27 (4%) |
| Left ventricular ejection fraction | 95 (24%) |
| Indication for oral anticoagulation | |
| Atrial fibrillation | 367 (58%) |
| Deep vein thrombosis/pulmonary embolism | 60 (10%) |
| Mechanical heart valve | 45 (7%) |
| Dilated cardiomyopathy | 43 (6%) |
| Ischemic heart disease | 26 (4%) |
| Cardiac thrombus | 20 (3%) |
| Previous stroke/transient ischemic attack | 15 (2%) |
| Biologic heart valve | 4 (1%) |
| Left ventricular aneurysm | 4 (1%) |
| Other | 48 (8%) |
| Indication for percutaneous coronary intervention | |
| ST-segment elevation myocardial infarction | 108 (17%) |
| Non–ST-segment elevation acute coronary syndromes | 294 (46%) |
| Other | 231 (37%) |
| Number of treated coronary arteries | |
| 1 | 505 (80%) |
| ≥2 | 127 (20%) |
| Type of stent | |
| Drug eluting | 156 (25%) |
| Bare metal | 449 (71%) |
| Other | 27 (4%) |
| Use of glycoprotein IIb/IIIa inhibitors | 96 (25%) |
The antithrombotic therapy most frequently prescribed at discharge was DAPT ( Table 2 ).
| Variable | Value |
|---|---|
| DAPT | 306 (48%) |
| TT (OAC, aspirin, and clopidogrel) | 205 (32%) |
| OAC and aspirin | 111 (18%) |
| Other | 10 (2%) |
The cumulative 1-year occurrence of the combined outcome, including MACVEs and major bleeding, was 27% ( Table 3 ). This rate was driven mainly by target vessel revascularization (12%) and cardiovascular death (10%), with the occurrence of stroke, stent thrombosis, and major bleeding limited to 2% to 3% ( Table 3 ).
| Variable | Value |
|---|---|
| Cardiovascular death | 61 (10%) |
| Myocardial infarction | 47 (8%) |
| Target vessel revascularization | 71 (12%) |
| Definite stent thrombosis | 12 (2%) |
| Stroke | 15 (3%) |
| Deep vein thrombosis/pulmonary embolism | 10 (2%) |
| Major bleeding | 16 (3%) |
| Combined (cardiovascular death, myocardial infarction, target vessel revascularization, definite stent thrombosis, stroke, deep vein thrombosis/pulmonary embolism, and major bleeding) | 167 (27%) |
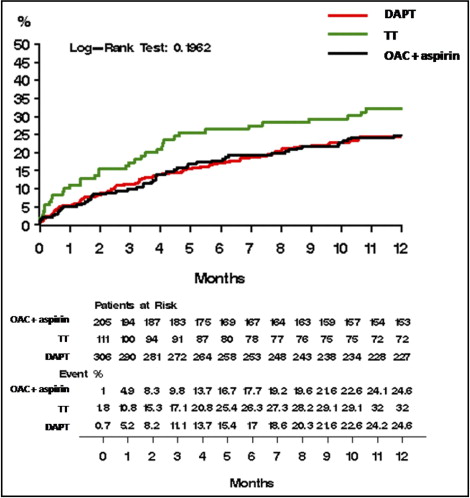
On analysis performed according to antithrombotic regimen, no significant differences were observed among TT, DAPT, and the combination of OAC and aspirin (32% vs 24.6% vs 24.6%, p = 0.19) on the 1-year cumulative occurrence of the combined outcome of MACVEs and major bleeding ( Figure 1 ) . Nor was the occurrence of cardiovascular death (9.9% vs 8.5% vs 10.2%, p = 0.78), myocardial infarction (11.3% vs 5.5% vs 9.3%, p = 0.08), target vessel revascularization (12.3% vs 10.3% vs 11.9%, p = 0.74), and venous thromboembolism (1.8% vs 2.8% vs 0%, p = 0.07) significantly different among the 3 treatment groups. The stroke rate was of borderline significance higher in the DAPT group compared to TT and OAC plus aspirin (4.1% vs 1.0% vs 1.1%, p = 0.057; Figure 2 ) . Stent thrombosis, although not significantly different among the 3 treatment groups, was highest with TT (2.7% vs 1.7% vs 2.0%, p = 0.77) ( Figure 3 ) . Of note, in the TT group, stent thrombosis was essentially confined to the periprocedural period, as opposed to the DAPT and OAC plus aspirin groups, in which it occurred throughout the follow-up period ( Figure 3 ). The incidence of major bleeding was highest, although not significantly different, in the TT group (5.0% vs 2.0% vs 2.6%, p = 0.32; Figure 4 ) . The increased occurrence of major bleeding was observed during the second 6 months of treatment, especially in the last 2 months, while it was comparable to that of DAPT and OAC plus aspirin during the first 6 months ( Figure 4 ).