Antiplatelet Therapy
Ashvarya Mangla, MD
Saurabh Gupta, MD
 General Considerations
General Considerations
Atherosclerosis is a leading cause of cardiovascular disease. Atherosclerotic plaques can acutely rupture, exposing a necrotic core that sets off the coagulation cascade, ultimately culminating in vascular occlusion. Activation of platelets is the initial event in this cascade and, depending on the vascular bed involved, can cause acute coronary syndromes, ischemic stroke, mesenteric ischemia, or acute limb ischemia. Antiplatelet therapy forms the core of treatment for both acute and chronic atherosclerotic disease. In this chapter, we will discuss antiplatelet therapy in the treatment of cardiovascular disease.
 Role of Platelets in Thrombosis
Role of Platelets in Thrombosis
After vascular injury, platelets are bound to exposed collagen and von Willebrand factor (vWF) and activated. Activated platelets then secrete thromboxane A2 (TXA2) and adenosine diphosphate (ADP), which leads to platelet aggregation and recruitment of more platelets. The final common pathway of platelet aggregation is mediated by glycoprotein (GP) IIbIIIa receptors that bind to fibrinogen and vWF, leading to platelet plug and clot formation. Antiplatelet agents target different pathways in this cascade (Figure 3–1).
 Classification of Antiplatelet Drugs (Figure 3–2)
Classification of Antiplatelet Drugs (Figure 3–2)
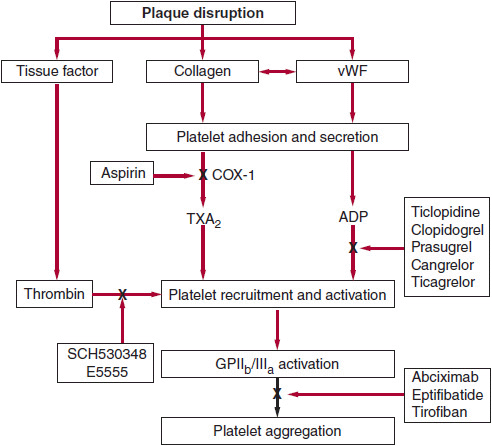
![]() Figure 3–1. Site of action of antiplatelet drugs. Aspirin inhibits thromboxane A2 (TXA2) synthesis by irreversibly acetylating cyclooxygenase-1 (COX-1). Reduced TXA2 release attenuates platelet activation and recruitment to the site of vascular injury. Ticlopidine, clopidogrel, and prasugrel irreversibly block P2Y12, a key adenosine diphosphate (ADP) receptor on the platelet surface; cangrelor and ticagrelor are reversible inhibitors of P2Y12. Abciximab, eptifibatide, and tirofiban inhibit the final common pathway of platelet aggregation by blocking fibrinogen and von Willebrand factor (vWF) binding to activated glycoprotein (GP) IIb/IIIa. SCH530348 and E5555 inhibit thrombin-mediated platelet activation by targeting protease-activated receptor-1 (PAR-1), the major thrombin receptor on human platelets. (Reproduced with permission from Longo DL, et al. Harrison’s Principles of Internal Medicine, 18th ed. New York: McGraw-Hill; 2012.)
Figure 3–1. Site of action of antiplatelet drugs. Aspirin inhibits thromboxane A2 (TXA2) synthesis by irreversibly acetylating cyclooxygenase-1 (COX-1). Reduced TXA2 release attenuates platelet activation and recruitment to the site of vascular injury. Ticlopidine, clopidogrel, and prasugrel irreversibly block P2Y12, a key adenosine diphosphate (ADP) receptor on the platelet surface; cangrelor and ticagrelor are reversible inhibitors of P2Y12. Abciximab, eptifibatide, and tirofiban inhibit the final common pathway of platelet aggregation by blocking fibrinogen and von Willebrand factor (vWF) binding to activated glycoprotein (GP) IIb/IIIa. SCH530348 and E5555 inhibit thrombin-mediated platelet activation by targeting protease-activated receptor-1 (PAR-1), the major thrombin receptor on human platelets. (Reproduced with permission from Longo DL, et al. Harrison’s Principles of Internal Medicine, 18th ed. New York: McGraw-Hill; 2012.)
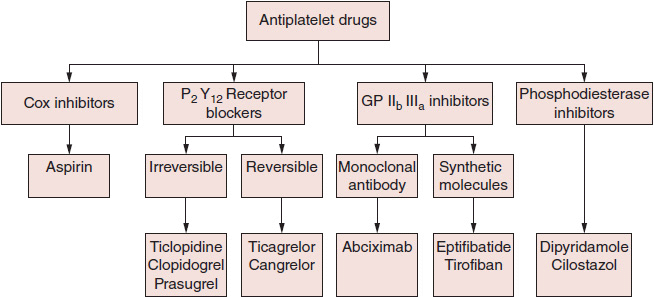
![]() Figure 3–2. Classification of antiplatelet drugs based on mechanism of action.
Figure 3–2. Classification of antiplatelet drugs based on mechanism of action.
A. Cyclooxygenase Inhibitors: Aspirin
1. Mechanism of action—Acetylsalicylic acid (ASA, aspirin) in low doses irreversibly inhibits cyclooxygenase-1 (COX-1), which is required for synthesis of TXA2, a vasoconstrictor required for platelet aggregation. At higher doses, ASA also inhibits COX-2, which is required for pros-tacyclin production; prostacyclins are inhibitors of platelet aggregation and vasodilators. Thus, for optimal antiplatelet effect, an ASA dose between 75 and 325 mg is recommended. For rapid onset of action, in ASA-naïve patients, a dose of at least 162 mg should be used.
2. Contraindications—ASA is contraindicated in patients with a history of bronchospasm or anaphylactic reaction.
3. Side effects—Dyspepsia, peptic ulcer, erosive gastritis, and upper gastrointestinal bleeding are dose-related side effects. Some patients may develop bronchospasm, urticaria, and rarely anaphylactic reactions. Complex acid–base abnormalities can occur in the setting of aspirin overdose. Some younger patients may have aspirin hypersensitivity with associated nasal polyps, allergic rhinitis, and bronchospasm (Samter’s triad). These patients may benefit from aspirin desensitization. Hyporesponsiveness to ASA is defined as the inability of ASA to produce expected inhibitory effects on platelet function. Clinically, this is associated with increased vascular events. Currently, there is no consensus on treatment, although empirically, an ADP receptor antagonist may be added.
4. Uses
A. ACUTE CORONARY SYNDROMES—All patients who present with chest pain and electrocardiographic or cardiac biomarker evidence of ST elevation myocardial infarction (STEMI) or non-STEMI (NSTEMI) should be treated with non–enteric-coated aspirin 162–325 mg (preferably crushed and chewed to enable rapid onset of action). Aspirin should be continued indefinitely in all patients who are not allergic at a dose of 75–162 mg/day, once daily. For patients allergic to aspirin, clopidogrel (or another P2Y12 receptor antagonist) is a reasonable alternative.
B. CHRONIC STABLE ANGINA—Aspirin 75–162 mg is a standard component of routine management of patients with chronic stable angina and has been demonstrated to reduce morbidity and mortality.
C. PERIPHERAL ARTERIAL DISEASE—Patients with symptomatic lower extremity peripheral arterial disease have a high likelihood of disease in other vascular beds and should be treated with aspirin to reduce risk of myocardial infarction (MI), stroke, or vascular death.
D. STROKE/TRANSIENT ISCHEMIC ATTACK (TIA)— Patients with prior ischemic stroke or TIA should receive aspirin 325 mg within the first 48 hours of onset of symptoms. If the patient has received thrombolytic therapy for ischemic stroke, antiplatelet therapy can be started after 24 hours. Therapy should be continued indefinitely at a dose of 75 or 81 mg orally once daily.
E. PRIMARY PREVENTION—Currently there is no evidence to support ASA for primary prevention in young patients (males age < 45, women age 55) or patients over the age of 80 years. Men aged 45–79 years (for reduction of MIs) and women aged 55–79 years (for reduction of ischemic strokes) should take low-dose aspirin for primary prophylaxis if their potential benefit exceeds the risk of GI bleed. Aspirin 75–162 mg/day can be considered in patients with type 1 and type 2 diabetes who have a > 1 10% 10- year risk of developing coronary artery disease (CAD) based on Framingham risk score.
B. P2Y12 Receptor Blockers
Commercially available products include the irreversible inhibitors ticlopidine, clopidogrel, and prasugrel and the reversible inhibitors cangrelor and ticagrelor.
1. Mechanism of action—These agents inhibit the P2Y12 receptor (required for binding ADP with a resultant increase in platelet aggregation and activation of GP IIbIIIa receptors on platelet surface). Inhibition of these receptors leads to decreased platelet activation and aggregation.
2. Contraindications—Active bleeding and a previous anaphylactic reaction are contraindications to therapy. Prasugrel is contraindicated in patients with a prior history of TIA or stroke and should be used with extreme caution in the elderly (≥ 75 years) or low body weight (< 60 kg) due to increase in the risk of bleeding events. Maintenance doses of aspirin above 100 mg appear to reduce the effectiveness of ticagrelor and should be avoided.
3. Side effects—Bleeding and purpuric lesions rarely occur. Ticlopidine can cause hematologic side effects such as bone marrow suppression, thrombocytopenia, thrombotic thrombocytopenic purpuras, and neutropenia, and regular blood count monitoring is recommended. This drug has largely been supplanted by alternatives with a more favorable risk–benefit ratio. Hematologic side effects are less common with clopidogrel and prasugrel. Skin rashes have also been reported with clopidogrel.
4. Clinically relevant pharmacology—Ticlopidine, clopidogrel, and prasugrel are prodrugs that require metabolism by liver to convert them into active drugs. After a loading dose, platelet inhibition starts in 4–6 hours. Prasugrel is absorbed more completely and has a relatively rapid onset of action. Nonthienopyridines, such as ticagrelor and cangrelor, are absorbed faster, have a more rapid onset, and have a shorter duration of action. In the setting of acute MI-STEMI or NSTEMI, when rapid inhibition of platelets is required, a loading dose of all antiplatelet agents is recommended.
5. Thienopyridine resistance—Some patients treated with adequate doses of clopidogrel still have thrombotic events despite medication compliance. This variability could partially be from genetic polymorphisms in the CYP isoenzymes involved in activation of clopidogrel. The prevalence of polymorphism is as high as 30–50% in certain populations. These genetic polymorphisms do not affect the activity of prasugrel, and it can be an alternative in populations suspected of having genetic predisposition to clopidogrel resistance. In the future, there may be a role for genetic testing prior to initiating clopidogrel.
6. Uses
A. ACUTE CORONARY SYNDROMES—For patients with STEMI, in whom primary percutaneous coronary intervention (PCI) is planned, P2Y12 blockers should be administered as a loading dose, before or at the time of PCI (clopidogrel 300–600 mg, prasugrel 60 mg, or ticagrelor 180 mg). In patients less than 75 years of age who receive fibrinolytic therapy or who do not receive reperfusion therapy, it is reasonable to administer an oral loading dose of clopidogrel 300 mg and continue long-term maintenance therapy (1 year). Prasugrel can be considered once coronary anatomy is known (typically after coronary angiography). Patients with NSTEMI/unstable angina who are selected for early invasive strategy should receive dual antiplatelet therapy including aspirin. The additional agent could be either a P2Y12 or a GP IIbIIIa inhibitor. Dual antiplatelet therapy should be continued for 12 months in patients with an acceptably low risk of bleeding.
B. POST PCI—Patients who undergo PCI should be on dual antiplatelet therapy with aspirin and any of the three P2Y12 inhibitors (clopidogrel, prasugrel, or ticagrelor) for 1 year.
C. ASA ALLERGY—Patients with chronic stable angina, peripheral arterial disease, or a stroke or TIA who are allergic to aspirin can be treated with clopidogrel. Dual antiplatelet therapy with aspirin and P2Y12 receptor antagonists is not indicated in patients without antecedent PCI, and dual anti-platelet therapy with aspirin and clopidogrel is not superior to either agent alone in patients with strokes or TIA.
C. GP IIbIIIa Inhibitors
The monoclonal antibody abciximab and the synthetic compounds eptifibatide and tirofiban are GP IIbIIIa inhibitors. Oral GP IIbIIIa receptor antagonists have not been demonstrated to be effective and currently have no role.
1. Mechanism of action—These drugs block the GP IIbIIIa receptors on the platelet surface. These are the most abundant receptors on the platelet surface and are the final common pathway of platelet aggregation and platelet plug formation.
2. Dosage—These agents are typically administered as an intravenous loading dose followed by a continuous infusion. The dose of abciximab does not need to be adjusted for patients with renal disease; however, tirofiban and eptifiba-tide are renally excreted, and the dose needs to be adjusted in patients with renal disease.
3. Contraindications—Abciximab should not be used in patients in whom PCI is not planned. Tirofiban and eptifibatide are contraindicated in patients with end-stage renal disease.
4. Side effects—Immune-mediated thrombocytopenia and bleeding are the common side effects. Thrombocytopenia is more common with the monoclonal antibody and relatively less common with synthetic agents. For patients being treated with these agents, daily monitoring of blood counts is recommended.
5. Uses: acute coronary syndromes—Patients who present with STEMI and are candidates for primary PCI (with or without stenting) are often treated with a strategy of heparin with a GP IIbIIIa inhibitor at the time of PCI or monotherapy with bivalirudin, in which case GP IIbIIIa inhibitors are reserved for patients with no reflow or with giant thrombus after PCI. In general, there is no role for GP IIbIIIa inhibitors after fibrinolytic therapy (regardless of whether fibrinolysis was successful) due to an increased risk for minor and major bleeding events. Patients with NSTEMI or unstable angina who are selected for early invasive strategy can be treated with GP IIbIIIa inhibitors in addition to aspirin either at the time of presentation or just before PCI. The agents of choice in this setting are eptifibatide and tirofiban. Patients for whom an early conservative strategy is chosen and who continue to have symptoms despite optimal medical therapy can also be considered for GP IIbIIIa inhibitors until the time of PCI.
D. Phosphodiesterase Inhibitors
Dipyridamole and cilostazol are phosphodiesterase inhibitors.
1. Mechanism of action—These agents inhibit the enzyme phosphodiesterase, thus increasing the concentration of cyclic adenosine monophosphate (cAMP) in platelets, which in turn inhibits platelet aggregation.
2. Uses
A. STROKE—Dipyridamole in combination with aspirin is superior to aspirin alone in secondary prevention of stroke.
B. PERIPHERAL ARTERIAL DISEASE—Patients with symptomatic lower extremity peripheral arterial disease and intermittent claudication in the absence of heart failure benefit from addition of cilostazol, with improvement in symptoms and increase in exercise tolerance.
C. CARDIAC STRESS TESTING—Due to its vasodilatory properties, dipyridamole is used in chemical stress testing.
 Treatment
Treatment
A. Special Issues with Antiplatelet Therapy
1. Gastrointestinal bleeding—If antiplatelet therapy is indicated in patients with a history of or those at increased risk for gastrointestinal bleeding (advanced age or concomitant warfarin, steroid, or nonsteroidal anti-inflammatory drug use), proton pump inhibitors should be used to prevent recurrence or for prophylaxis. Earlier concerns about diminished efficacy of clopidogrel with the use of proton pump inhibitors have not been validated in randomized clinical trials.
2. Management of patients receiving antiplatelet therapy who require a surgical procedure—Decision to withhold antiplatelet therapy should be individualized for each patient depending on the risk of bleeding and the indication for which the antiplatelet therapy is used. For patients receiving ASA and a P2Y12 receptor antagonist for a bare metal stent (BMS) who require urgent surgery within 6 weeks of placement, dual therapy should be continued in the perioperative period. For patients who are receiving ASA and clopidogrel for a drug-eluting stent (DES) and require urgent surgery within 12 months of placement, therapy should be continued in the perioperative period. Whenever possible, elective surgery in patients receiving ASA and clopidogrel secondary to coronary stent implantation should be deferred for at least 6 weeks after BMS placement and at least 12 months after DES placement.
3. Combined antiplatelet and anticoagulant therapy—Patients with CAD who are on anticoagulant therapy for atrial fibrillation or prosthetic heart valve and undergo PCI should be on dual antiplatelet therapy in addition to anticoagulant therapy. After PCI, the maintenance should consist of a combination of low-dose ASA (75–100 mg), warfarin, and clopidogrel (duration determined by the type of stent—1 month after BMS or 1 year after DES implantation). When warfarin is given in conjunction with clopidogrel or low-dose ASA, the dose-intensity must be carefully monitored. There is some evidence that a similar strategy of dual antiplatelet therapy with dabigatran may be safe with an acceptably low (albeit increased from baseline) bleeding risk, but caution is warranted until more data are available.
B. Special Populations
1. Antiplatelet therapy in atrial arrhythmias—In patients with atrial fibrillation younger than 60 years without heart disease or risk factors for thromboembolism (lone atrial fibrillation), ASA for primary prevention of stroke is probably reasonable, although the effectiveness of this approach is not well established. For patients at low risk for thromboembolism (age 65–74, female sex, CAD), antiplatelet therapy with aspirin 75–325 mg is a reasonable alternative to anticoagulation. Patients who have just one moderate risk factor for thromboembolism (hypertension, age > 75 years, congestive heart failure, ejection fraction < 35%, diabetes mellitus) may be candidates for antiplatelet therapy with ASA 75–325 mg alone if their risk of bleeding from antithrombotic agent is high, because of patient preference, or if there are issues with sustained monitoring of anticoagulation. Patients with atrial fibrillation or atrial flutter who have more than one moderate risk factor and cannot take anticoagulant therapy for reasons other than bleeding may be started on dual antiplatelet therapy with ASA and clopidogrel, although this therapy is inferior to anticoagulant therapy.
2. Coronary artery bypass grafting (CABG)—When possible, ASA should be administered preoperatively to patients in whom CABG is planned; if not initiated preoperatively, it should be started within 6 hours of surgery and continued indefinitely to prevent graft occlusion. For patients who are intolerant or allergic to aspirin, clopidogrel 75 mg orally once daily can be substituted. Patients on dual antiplatelet therapy with aspirin and P2Y12 receptor blockers scheduled to undergo elective CABG should discontinue clopidogrel and ticagrelor at least 5 days and prasugrel at least 7 days prior to surgery. For urgent surgery, these agents should preferably be discontinued at least 24 hours before surgery to reduce the risk of major bleeding. The GP IIbIIIa inhibitors eptifibatide and tirofiban should be discontinued at least 2–4 hours before surgery and abciximab at least 12 hours before surgery.
3. Pregnancy—Antiplatelet agents can be considered in pregnancy or breastfeeding if maternal benefits clearly outweigh potential fetal risks. Low-dose ASA (75–162 mg daily) is likely safe for use during the first trimester of pregnancy; however, it should be avoided during the third trimester and while breastfeeding. Use of agents other than low-dose ASA should only be considered after weighing maternal benefits with potential risks for the newborn. P2Y12 receptor antagonists should be used during pregnancy or breastfeeding only if clearly indicated.
4. Patients with prosthetic heart valves—The addition of daily ASA (75–100 mg) to therapeutic warfarin is recommended for all patients with mechanical heart valves and those patients with biological valves who have risk factors. For patients with an aortic or mitral bioprosthesis and no risk factors, aspirin is indicated at 75–100 mg/day. For patients unable to take warfarin, ASA is indicated in a dose of 75–325 mg/day (or clopidogrel if intolerant or allergic to ASA).
5. PCI—After PCI, ASA should be continued indefinitely at a dose of 75 or 81 mg once daily. Typically a P2Y12 inhibitor is indicated for at least 12 months regardless of the stent type. In select patients after BMS implantation and an increased risk of bleeding, dual antiplatelet therapy maybe continued for 1 month. Options for P2Y12 inhibitors include clopidogrel 75 mg daily, prasugrel 10 mg daily, or ticagrelor 90 mg twice daily.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


