CHAPTER 41 Anterior Mediastinal Masses
Although the anatomy of the mediastinum is presented in detail elsewhere in this book, it is reviewed here from a thoracic surgical perspective, to highlight the radiographic and surgical anatomic subdivisions of the mediastinum that have been proposed. The simplest and most commonly used description is the three-compartment model proposed by Shields.1 He divided the mediastinum into the anterior compartment, the middle (or visceral) compartment, and the posterior (or paravertebral) compartment. The anatomic limits of each compartment are shown in Figure 41-1, and the structures contained within each compartment are listed in Table 41-1. Locating a mass in the anterior mediastinum allows a differential diagnosis to be generated based on the knowledge of the normal structures in that compartment. From the perspective of thoracic surgery, this approach best allows the appropriate diagnostic and therapeutic procedures to be selected.
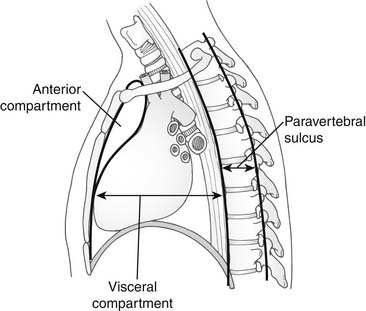
Figure 41–1 Three-compartment division of the mediastinum as proposed by Shields.
(From Shields TW. General thoracic surgery. 2nd ed. Philadelphia: Lea & Febiger; 1983, with permission.)
Table 41–1 Components of Mediastinal Compartments∗ as Proposed by Shields
| Anterior | Visceral (middle) | Paravertebral (posterior) |
|---|---|---|
| Thymus | Pericardium/heart | Sympathetic chain |
| Internal thoracic vessels | Great vessels | Proximal intercostals: nerve, artery, and vein |
| Internal thoracic lymph nodes | Trachea | Posterior paraesophageal lymph nodes |
| Prevascular lymph nodes | Proximal right and left main-stem | Intercostal lymph nodes |
| Fat and connective tissue | Esophagus | |
∗ The nodal basin draining the anterior chest wall and female breast lie in the anterior compartment, whereas the majority of those draining the lung and important in lung cancer staging lie in the visceral compartment.
From Shields TW. General thoracic surgery. 2nd ed. Philadelphia: Lea & Febiger; 1983, with permission.
Table 41-2 is an extensive list of the pathologies that can appear as a mass in the anterior mediastinal compartment. By far, the four most common are thymoma, lymphoma, teratoma, and germ cell tumor.
Table 41–2 Mass Lesion in the Anterior Mediastinal Compartment: Differential Diagnosis
| Neoplastic |
| Infectious |
| Vascular |
THYMIC TUMORS
Lesions of the thymus account for approximately 50% of anterior mediastinal masses in adults and are thus of great importance. Although lymphomas and germ cell tumors of the anterior mediastinum often involve the thymus or actually arise from cells within the thymus (or both), these are most appropriately discussed as a group separate from thymic tumors and are usually classified separately. The thymic masses discussed in this section, then, are listed in Box 41-1.
Thymoma
Thymomas may be completely encapsulated or invasive. Large series have demonstrated the incidence of encapsulated lesions to be between 40% and 70% and that of microscopic or grossly invasive lesions to be between 30% and 60%. Although local invasion is usually limited to the capsule or to immediately adjacent structures, spread to more distant sites within the chest does occur, particularly to the pleura, diaphragm, and mediastinal lymph nodes. Aggressive thymomas have been reported to be associated with distant metastases in as many as 30% of patients at high-level referral centers,2 but most broad studies report distant metastasis to be rare (<5%).
Pathology
Thymomas are derived from thymic epithelial cells, but most contain varying mixes of epithelial cells and lymphocytes.3 Traditional histologic classifications have therefore grouped thymomas according to cytologic make-up: (1) predominantly lymphocytic, (2) predominantly epithelial, and (3) mixed.4 There is also a recognized spindle cell variant of the epithelial subtype. Approximately 50% of the tumors are of the mixed variety, with the remainder split between the epithelial and lymphocytic subtypes. Unfortunately, these subtypes have little prognostic significance, other than the generally better prognosis of the spindle cell variant, and thus more recent investigators have proposed alternative histologic classification schemes (see later).
Of clinical significance is the fact that it may be more difficult to establish the diagnosis of thymoma from a small sample (e.g., by needle biopsy) of a lymphocyte-predominant thymoma versus the other subtypes because of its microscopic similarity to lymphoma. Immunohistochemical staining for cytokeratin often helps in making the diagnosis, as antibodies to this protein are present in 95% to 100% of thymomas.5 Chromogranin staining allows differentiation between thymoma and thymic carcinoid (the former being negative and the latter positive for chromogranin).
Classification
No TNM classification has been found to be of value for staging thymomas.
The most widely used clinical classification scheme is that proposed by Masaoka and colleagues in 19816 (Table 41-3). This scheme takes into account the gross presence or absence of encapsulation and fixation or invasion into adjacent structures as identified at the time of surgery. It also recognizes the fact that a grossly well-encapsulated tumor may be found to have invasion through the capsule at microscopic examination. In the years since it was originally proposed, the Masaoka system or later variations of it have been found to have prognostic significance by numerous authors.
Table 41–3 Classification Schemes for Thymoma
| Masaoka | WHO∗ | |
|---|---|---|
| Stage I | Encapsulated, tumor may invade into, but not through capsule microscopically. | Type A (spindle cell, medullary) |
| Stage II | Type AB (mixed) | |
| IIa | Macroscopic invasion into thymus or fat or adherent to but not through pleura or pericardium | Type B |
| IIb | Microscopic trans-capsular invasion | B1 (lymphocyte-rich, predominantly cortical) |
| Stage III | Macroscopic invasion of neighboring organs (pericardium, great vessels, lung) | B2 (cortical) |
| Stage IV | B3 (epithelial, well-differentiated thymic carcinoma) | |
| IV A | Pleural or pericardial dissemination | Type C (thymic carcinoma) |
| IV B | Lymphogenous or hematogenous mets | |
∗ In the World Health Organization (WHO) column, terms in parentheses represent nomenclature from previous histologic classifications that most closely approximate the current classification category.
In 1985, Marino and Muller-Hermelink7 proposed a histologic classification that has come to be known as the Muller-Hermelink (MH) classification. This scheme divides thymomas into cortical, medullary, and mixed types. The cortical type contains medium to large epithelial cells of characteristic appearance, and usually abundant lymphocytes. The medullary type contains small to medium cells with different features and fewer lymphocytes. The former tend to be of higher clinical stage, whereas the latter tend to be of lower invasiveness. Many investigators have generated data in support of the prognostic significance of the MH classification,8–11 showing that the medullary type has the best and the cortical type the least favorable prognosis. The World Health Organization (WHO) has adopted a classification system based on the MH criteria (see Table 41-3).12 It has been suggested that such histologic systems be used in combination with a Masaoka-type staging system to provide more precise prognostic information.
Association with Myasthenia Gravis
Patients with thymomas may present with a number of associated diseases, largely autoimmune in etiology, but the most common associated illness is myasthenia gravis. Accumulated experience suggests that 5% to 15% of patients with MG are found to have thymomas, and that 30% to 50% of thymomas are associated with clinical MG. Notably, the disease may develop later, even after thymoma resection, if it is not present at the time of discovery of the thymic tumor. For this reason, it is essential that a complete thymectomy be performed as part of the resection of any anterior mediastinal tumor that may be a thymoma. As MG is an autoimmune disease caused by anti-acetylcholine receptor (anti-AChR) antibodies, its relationship to thymoma and treatment by thymectomy appears to be related to the role of the thymus in the creation of these antibodies.13,14
This association has a long and very interesting history that is beyond the scope of this chapter. Highlights, however, include the first description by Schumacher and Roth in 1912 of improvement in MG after thymectomy15; the more systematic evaluation of this concept by Blalock and coworkers in the late 1930s16; and the subsequent controversy over whether the morbidity and mortality of the procedure justified the chances of a remission from MG. Only in the late 1960s and 1970s did thymectomy for MG in the absence of thymoma gain wide acceptance, as improvements in perioperative care reduced the morbidity of the procedure and the benefits became clearer. Although convincing data suggest that patients with MG who are treated with thymectomy have a higher remission rate than historical controls treated by medication alone, there has never been a randomized study to definitively establish this concept. Just such a study is, however, now ongoing, sponsored by the National Institute of Neurological Disorders and Stroke.
Association with Other Diseases
Box 41-2 lists MG and the other systemic, autoimmune disorders most commonly associated with thymoma. Two percent to 15% of patients with thymoma suffer from some type of cytopenia. The most common type is pure red cell aplasia, thought to result from an abnormal IgG antibody that inhibits red cell synthesis. Most patients with this disease have the favorable, spindle-cell type of thymoma.17 Approximately one third of patients with aplasia demonstrate improvement after thymectomy.18 Hypogammaglobulinemia occurs in less than 5% of patients with thymoma, principally older patients. This disease generally does not respond to thymectomy, and the prognosis is poor. Of the other autoimmune disorders that occur in association with thymoma in lower frequencies, lupus appears to be the most common; again, resection does not appear to have an impact on the clinical course.
The Anterior Mediastinal Mass That Might Be Thymoma
Imaging
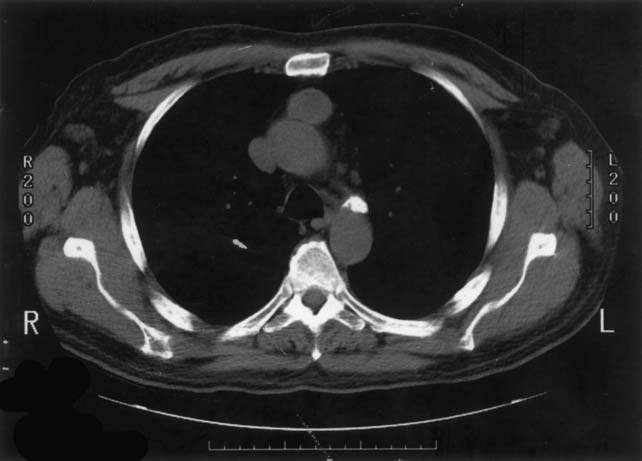
Although no CT appearance is diagnostic of thymoma, a well-circumscribed, solid anterior mediastinal mass in an adult older than 40 years, without low-density areas that suggest the cystic and fatty components of a teratoma, is very likely to be a thymoma (Figs. 41-2 and 41-3). The presence of calcification is not particularly helpful, as both thymomas and teratomas may contain calcium. In some cases, lymphoma will be the obvious diagnosis on the basis of adenopathy outside the anterior mediastinum, but in the absence of this finding, differentiating a thymoma from a lymphoma may be difficult. It is often possible to suspect one over the other only on the basis of the thymoma patient’s typically more advanced age. Magnetic resonance imaging (MRI) provides useful additional information only when the fluid or fatty nature of a component of the tumor is not clearly defined by CT, or when a vascular structure (e.g., an aneurysm) is suspected and has not been clearly ruled out by the contrast CT study.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree