Chapter 39 Aneurysms of the Aorta and Iliac Arteries
Aneurysms of the abdominal aorta are common. The incidence (the number of new cases) has been increasing for more than 3 decades, having tripled since 1970. The increased incidence has been noted in the United States and other Western countries; it is due not only to the aging of the population and improved diagnostic methods, but also to an absolute increase in the number of new cases.1–4 It is estimated, from large national health screening programs, that 1.1 million Americans have this condition—a prevalence of 1.4% in the 50- to 84-year-old population.5
Approximately 190,000 new cases are diagnosed and more than 50,000 repairs are performed annually. The incidence and prevalence vary, depending on a number of factors, including the population studied; it is lowest in unselected groups and higher in patient groups with other atherosclerotic lesions (Table 39-1).5–10 In a study at Massachusetts General Hospital, abdominal aortic aneurysms were found in 2% of 24,000 consecutive autopsies.6 In a more recent autopsy series from Malmö, Sweden, abdominal aortic aneurysms were found in 4.3% of men and 2.1% of women.6 This last study, as well as others, was performed on an almost entirely white population, and it is known that aneurysms are most common in white males. Aneurysms were found in 5.9% of male smokers older than 55 years in a U.S. Department of Veterans Affairs (VA) screening program.8 The prevalence in screening studies from Asian and African countries is much lower, because it is among African and Hispanic Americans (ratio of white to African American is 3.5 : 1). The male-to-female ratio is 4 : 1 to 5 : 1 in the 60- to 70-year-old group, but beyond 80 years old the ratio approaches 1 : 1. The frequency of aneurysms increases steadily in men older than 55 years, reaching a peak of 5.9% at 80 to 85 years. In women, there is a continuous increase in prevalence after the age of 70 years, reaching a peak of 4.5% at age greater than 90 years. In community screening programs, the prevalence in men 65 to 74 years old ranges from 2.7% to 3.4%, whereas in elderly hypertensive men and women, the prevalence has been reported as high as 10.7% to 12%.11–13 The increased incidence and prevalence noted over the past 3 decades has occurred in both men and women during a time when the incidence of death from coronary artery and other forms of atherosclerosis has been decreasing.
TABLE 39-1 Incidence of Abdominal Aortic Aneurysms
| Category | Incidence (%) |
|---|---|
| Autopsy | 1.5-3.0 |
| Unselected patients screened by ultrasonography | 3.2 |
| Selected patients with CAD | 5.0 |
| Selected patients with PVD | 10.0 |
| Patients with femoral or popliteal aneurysms | 50.0 |
CAD, Coronary artery disease; PVD, peripheral vascular disease.
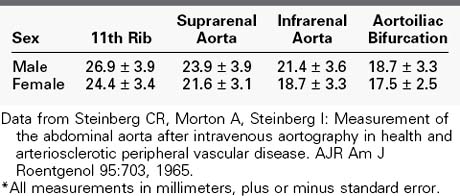
There is disagreement about the definition of an aneurysm, and several formulas have been proposed. The ad hoc committee on reporting standards of the Society for Vascular Surgery (SVS) and the International Society for Cardiovascular Surgery (North American chapter) defined an aneurysm as a permanent localized dilatation of an artery with an increase in diameter of greater than 50% (1.5-fold) its normal diameter.14 This ratio accounts for the normal variability in aortic diameter owing to several factors, including age, sex, and blood pressure. The aortic diameter increases steadily with age. As a result, the infrarenal aortic diameter in a 75-year-old person can vary from 12.4 mm in a small woman to 27.6 mm in a large man.15 An aortic diameter of 30 mm might not meet the definition of 50% increase in some people, but this value has been chosen as the definition for a number of studies on the natural history of small aneurysms and has been adopted by both the Society for Vascular Surgery and the European Society for Vascular Surgery because it is more than 2 SD above the mean for both men and women.16,17 The normal sizes of the aorta in adult males and females are listed in Table 39-2.14,15 Generalized dilatation of an arterial segment is frequently present in patients with aneurysms. When the diameter is increased less than 50% above normal, this is termed ectasia, whereas arteriomegaly represents diffuse enlargement of the arterial tree, but not large enough to meet the definition of aneurysm.18 In some patients with abdominal aortic aneurysms, the entire aortoiliofemoral arterial tree is arteriomegalic or ectatic. Arteriomegaly is an interesting condition caused by a systemic alteration in the elastic components of the arterial wall. It was seen in approximately 5% of nearly 6000 patients undergoing arteriography in one series, and there were discrete aneurysms in at least three different locations in about one third of them. All were men who were approximately 5 years younger than those with solitary aortic aneurysms.19
Aneurysms of the infrarenal aorta are by far the most common arterial aneurysms encountered in clinical practice. They occur three to seven times more frequently than thoracic aneurysms. Men are affected more than women by a ratio of 4 : 1.8 Other aneurysms frequently coexist in patients with aortic aneurysms, including common or internal iliac aneurysms (in 20% to 30% of patients) and femoropopliteal aneurysms. Up to 85% of patients with femoral artery aneurysms have an aortic aneurysm. Popliteal aneurysms are also markers of abdominal aortic aneurysms. Aortic aneurysms can be found in approximately 8% of patients with a unilateral popliteal aneurysm, but in up to 60% of patients who have bilateral popliteal aneurysms. In at least one group of patients with carotid atherosclerosis, there was a 10% incidence of abdominal aortic aneurysm, and a 40% incidence of aortic aneurysms was found in another group of patients with tortuous internal carotid arteries.19,20 Overall, multiple aneurysms occur in 3.4% to 13% of patients with thoracic aneurysms in approximately 12% of those with abdominal aortic aneurysms.21,22
Screening
There have been several recent large ultrasound-based screening programs involving over 149,000 persons to detect aortic aneurysms.23,24 Aortic aneurysms were found in 4.9% to 7.6% of ultrasound-screened British men older than 65 years, but only in 1.3% of women. Similar data has been reported from Denmark, Australia, the Netherlands, and Norway. Although most screen-detected aneurysms are small (<4 cm) screening has been shown to be cost-effective and to reduce aneurysm-related, but not all-cause, mortality, at least in men. Because it is impractical and financially unfeasible to screen all adults, screening programs have been designed based on known aneurysm-related risk factors. Cigarette smoking correlates with the presence of aortic aneurysms, with an 8 : 1 preponderance of aneurysms in smokers compared with nonsmokers.25–27 Aortic aneurysms have been detected by ultrasound screening in 8.8% of male smokers older than 65 years who have peripheral vascular occlusive disease. There is also a well-recognized familial component to aortic aneurysms, present in 15% to 25% of cases. The current SVS Guidelines recommend screening for all men 65 years of age or older and for women 65 years and older who have smoked or have a family history of aortic aneurysm. These recommendations are somewhat more inclusive than those of the United States Preventive Services Task Force that did not include women without a family history or men who had never smoked. Nevertheless, this leads to Medicare offering ultrasound screening to men 65 to 75 years old who have smoked at least 100 cigarettes as well as in both men and women of the same age with a family history of aneurysm. It is reasonable to also recommend screening for all first-degree relatives of any patient with this diagnosis, based on the known genetic relationships.28
Pathogenesis of Aortic Aneurysms
There are several well-known but uncommon causes of abdominal aortic aneurysm, including cystic medial necrosis, dissection, Ehlers-Danlos syndrome, HIV, and syphilis. More than 90%, however, are associated with atherosclerosis, which has traditionally been considered the primary cause.29 Although aneurysms and occlusive atherosclerosis share most of the same risk factors (e.g., aging, tobacco use, hypertension, male gender, hypercholesterolemia) and atherosclerosis is uniformly present in the wall of aortic aneurysms, it is believed that several factors in addition to atherosclerosis are involved in aneurysm development.30–34 One observation that casts doubt on atherosclerosis being the sole cause of aortic aneurysms is that most patients with aneurysmal disease do not have occlusive vascular disease involving the aortoiliofemoral segments. It has been estimated that no more than 25% of aortic aneurysms are associated with significant occlusive disease. Another factor is the negative association of diabetes with aneurysms in contrast to its strong relationship with occlusive vascular disease. In addition, induction of aneurysms in animals fed an atherogenic diet has not been predictable, although regression of experimental atheromas has led to aneurysm formation in monkeys. These plus other observations have led to the concept that atherosclerosis is either a coincidental or a facilitating process rather than the primary cause and that complex biological processes are responsible for the destruction of the media of the aortic wall that characterizes aneurysms.
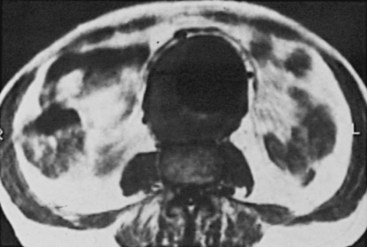
Mature elastin and collagen are the major structural proteins responsible for the integrity of the aortic wall. Collagen composes approximately 25% of the wall of an atherosclerotic aorta, but only 6% to 18% of an aneurysmal aortic wall. Biochemical studies have shown decreased quantities of both elastin and collagen, but an increased ratio of collagen to elastin in the walls of aneurysms.35,36 Elastin fragmentation is the initial structural event in aneurysm formation, and elastin depletion is complete early in aneurysm development. This has been correlated with the histopathologic features of a thin, dilated wall with fragmentation of elastin in the media, its replacement by a much thinner layer of collagen (mostly types I and III), loss of smooth muscle cells, and remodeling of the extracellular matrix. This thinned wall usually contains calcium and atherosclerotic lesions, rendering the wall brittle. Laminated thrombus lines the lumen, often eccentrically, resulting in a nearly normal flow channel (Figures 39-1 to 39-3; see color plate for Figure 39-3C), but possibly making the inner layers of the aortic wall relatively hypoxic. Aneurysms elongate as they dilate, causing them to become bowed and tortuous. It is believed that the weakening and fragmentation of the elastic lamellae is what permits vessels to lengthen excessively and become tortuous. As a result, the failure of elastin to provide sufficient retractive force in both the circumferential and longitudinal directions allows for increased aneurysm diameter and length, respectively.
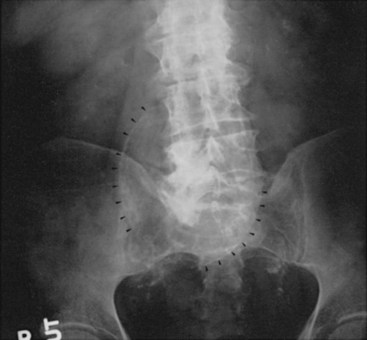
FIGURE 39-1 Plain abdominal radiograph showing large aortic aneurysm with calcified rim (arrowheads).
The aortic wall is composed of lamellar units that consist of collagen (mainly types I and III) and elastin, as well as vascular smooth muscle cells. There are more lamellar units in the thoracic (58) than in the abdominal aorta (40), and there is a further abrupt decrease below the renal arteries (26). This factor is believed to have a role in the predilection for aneurysms to develop in the terminal portion of the aorta combining with the fragmentation of the elastin and the overall thinning of the wall to contribute to its weakening.27 The large loss of elastin is one of the most consistent biochemical and histochemical findings in human aortic aneurysms.37,38 A chronic inflammatory cell infiltrate is also prominent. A key unresolved question is what triggers this inflammatory reaction and the subsequent chain of events. Many theories have been proposed, including a reaction to mural atherosclerotic plaque or a latent infectious process (e.g., Chlamydia pneumoniae or oral flora).39–41
These well-established histologic features have prompted a search for nonatherogenic mechanisms that disrupt collagen and elastin in the aortic wall. Several investigators have found excessive collagenase (matrix metalloproteinase [MMP]-1, -2, -3) activity in the wall of aneurysmal aortas, and others have found increased elastase (MMP-9) activity. These are members of a family of MMPs that are believed to have an essential role in aneurysm formation. MMP-9 is found in abundance in medial smooth muscle cells as well as in inflammatory cells, and increased levels have been found in both the aortic wall and serum in up to 50% of patients with aortic aneurysms, but not in those with aortic occlusive disease. These increased serum levels decline to normal after aortic aneurysm repair. Increased activity of other matrix proteases in aneurysmal aortic tissue has also been reported, as has an increased leukocyte-derived elastase in the blood of smokers with aneurysms. Deficiencies in antiproteases, such as several tissue inhibitors of metalloprotease and α1-antitrypsin, have also been described. α1-Antitrypsin is one of the most important natural antagonists to elastase and is responsible for the association between chronic obstructive pulmonary disease (emphysema patients with reduced α1-antitrypsin levels) and increased prevalence of aortic aneurysm and increased rate of rupture. Another factor is the chronic inflammatory infiltrate that occurs in the outer layers of aneurysmal aortas, consisting of macrophages and T and B lymphocytes. There are also increased levels of cytokines, immunoglobulin (Ig) G, and IgM that are not seen in association with aging or with occlusive aortic lesions. These findings may be the explanation for the increased levels of serum inflammatory markers, such as Il-6, C-reactive protein, neutrophil elastase, and others found in patients with aneurysms but not in those with occlusive disease. These inflammatory cells and cytokines are believed to interact in some as yet unexplained way with the connective tissue cells and matrix proteins in the pathogenesis of aneurysms.39,40 Most of the research has focused on the aortic media, but the role of the adventitia in aneurysm formation has recently received some interest. Normally, the adventitia is thought to limit maximal aortic diameter. Topical application of elastase to the adventitia leads to aneurysm formation in experimental animals solely owing to degradation of elastin.42
There is also considerable evidence that there is a genetic susceptibility to aortic aneurysm formation.43–49 Several investigators have discovered genetically linked enzyme deficiencies that are associated with aneurysms in experimental animals. For example, Tilson and Seashore45,46 showed that a deficiency in the copper-containing enzyme lysyl oxidase is the cause of aortic aneurysms in a strain of mice. Lysyl oxidase is important in collagen and elastin cross-linking, and this enzyme defect is sex chromosome linked. In addition, several reports of familial clustering of abdominal aneurysms support the notion of a genetic predisposition to this disease. Approximately 20% to 29% of patients with abdominal aneurysms have a first-order relative with the same condition.49 The age- and sex-adjusted increased risk of having an aneurysm is 11.6 times, according to one report. Female siblings are at particularly high risk. The genetic pattern of increased susceptibility is yet to be completely worked out. Available evidence supports both X chromosome–linked and autosomal dominant patterns of inheritance. Among the mutant suspect genes are the type III collagen gene (COL3A1) and the fibrillin gene (on chromosome 15). So-called familial aneurysms do not appear to be anatomically or clinically distinguishable from those with no familial pattern, except that they develop earlier in life, have a decreased male-to-female ratio of 2 : 1, and appear to have an increased risk of rupture. The clinical implications of these genetic studies, as limited as they are, strongly support the screening of all first-degree relatives of patients with abdominal aortic aneurysms.
Hemodynamic (mechanical) factors may also contribute to aneurysm development. The abdominal aorta is subjected to large pulsatile stresses as a result of its tapering geometry, relatively increased stiffness distally, and the reflected pressure waves from the peripheral vessels. Reductions in the number of elastic lamellae and the virtual lack of vasa vasorum in the media of the distal abdominal aorta may also be factors favoring aneurysmal formation in this segment of the arterial tree, making the aorta structurally less capable of handling the increased hemodynamic stresses that occur there.32
Aneurysm Enlargement
Once an aneurysm develops, it tends to enlarge gradually yet progressively. The enlargement rate has been well studied and averages 0.2 to 0.3 cm/yr for small aneurysms (3.0 to 5.5 cm diameter) to 0.3 to 0.5 cm/yr for larger aneurysms.50 Unfortunately the growth rate is nonlinear, making it impossible to predict the rate of enlargement of any individual aneurysm. Some aneurysms remain the same size for many years, whereas others enlarge rapidly. Factors associated with more rapid growth include larger initial size, hypertension, pulse pressure, active smoking, and cardiac or renal transplant. Interestingly, diabetes mellitus does not appear to be a factor in aneurysm development or growth. Once an aneurysm develops, regardless of the cause, its enlargement is governed by physical principles, especially Laplace’s law, which describes the relationship between the tangential stress (T) tending to disrupt the wall of a sphere, the radius (R), and the transmural pressure (P): T = PR. Thus, for a given transmural pressure, the wall tension is proportional to the radius. Once dilatation of the aorta has started, Laplace’s law explains why aortic enlargement is enhanced. It also explains why large aneurysms are more prone to rupture than small ones and why hypertension and pulse pressure are important risk factors for rupture. Using Laplace’s law, tripling the aortic radius from 2 to 6 cm results in a more than threefold increase in wall tension, and when this tension exceeds the tensile strength of the collagen in the aortic wall, disruption occurs. Although Laplace’s law has long been used as the sole explanation for aneurysm enlargement and rupture, it is at best an imperfect explanation because it was derived for perfect cylinders and spheres, and aortic aneurysms do not have uniform shape; it also does not consider the wall thickness, which can be highly variable. In addition, it does not explain why all aneurysms of the same diameter do not rupture or why some small aneurysms rupture and some large ones do not. Aneurysms usually do not rupture at the point of greatest diameter, as would be predicted by Laplace’s law. Recent studies using finite element analysis of computed tomography (CT) scan–derived data have measured wall stress and strain patterns and have shown that, in addition to decreased tensile strength, aneurysm walls have increased wall stress, an asymmetrical shape, and a complex radius (actually many radii) of curvature.51–55 Wall stress varies with shape and thickness, and the points of maximal stress do not necessarily coincide with the location of maximal diameter. They do, however, correspond closely with the location of rupture. Wall stress data might also explain why eccentric and saccular aneurysms have a greater risk of rupture than those with smooth fusiform shapes. The influence of calcification and mural thrombus on stress patterns is uncertain but may be important. These stress analyses have been validated by several investigators and may become a useful method for predicting risk of aneurysm rupture.
There has been wide interest in pharmacotherapy to reduce the rate of enlargement of small aortic aneurysms and thereby reduce rupture risk. β-Blockers have been studied in several randomized clinical trials with disappointing negative results. Similar results have been found with doxycycline and roxithromycin as well as angiotensin-converting enzyme inhibitors. Statins, however, have been found to reduce expansion rate and rupture risk. Unfortunately most of the studies with all these agents were not well designed or had insufficient statistical power to permit definitive conclusions, but it seems prudent for patients with small aortic aneurysms to be taking a statin drug for this purpose.16,17
Clinical Manifestations
Seventy percent to 75% of all infrarenal abdominal aortic aneurysms are asymptomatic when first detected.56 Detection can occur during a routine physical examination, but most often occurs during an imaging study performed for some other reason (e.g., upper gastrointestinal series, barium enema, intravenous pyelography, lumbosacral spine radiography, or abdominal CT or ultrasound examination). Occasionally, an aneurysm is first discovered during an unrelated abdominal operation.
Abdominal aortic aneurysms can cause symptoms as a result of rupture or expansion, pressure on adjacent structures, embolization, dissection, or thrombosis.56,57 Compression of adjacent bowel can cause early satiety and even nausea and vomiting. Because the duodenum crosses in front of the aorta, it is the part of the bowel most frequently compressed. Virtually any type of abdominal, flank, or back pain can be caused by an aneurysm. This fact often leads to delays in diagnosis. Chronic abdominal or back pain is the most common symptom, occurring in up to one third of patients. Large aneurysms can actually erode the spine and cause severe back pain, even in the absence of rupture.
The abrupt onset of severe pain in the back, flank, or abdomen is characteristic of aneurysmal rupture or expansion. It is uncertain why pain is produced by an expanding but unruptured (intact) aneurysm. The best explanation is sudden stretching of the layers of the aortic wall, with pressure on adjacent somatic sensory nerves or overlying peritoneum. Tenderness of the palpated aneurysm suggests that abdominal symptoms are arising from the aneurysm, although tenderness by itself is not a reliable indicator of impending rupture. In most surgical series, symptomatic but unruptured aneurysms account for 6% to nearly 40% of cases (average of five series totaling 311 patients: 13.7%).58 The timing of surgical treatment is made more difficult in these cases. A good-quality CT scan can show no sign of rupture but cannot predict when rupture might occur.
Ruptured aneurysms constitute between 20% and 25% of most series. The presence of an aneurysm is known in 25% to 33% of patients before rupture occurs. The nature of symptoms and their time course vary depending on the nature of the rupture.58–60 Small tears of the aneurysmal sac can result in a small leak that temporarily seals with minimal blood loss; this is usually followed within a few hours by frank rupture, which produces a catastrophic medical emergency. Rupture most frequently occurs through the posterolateral aortic wall on the left side into the retroperitoneal space; less commonly, it occurs through the anterior wall into the free peritoneal cavity. The incidence of this latter type of rupture is higher than indicated in most surgical series, because most of these patients die before reaching the hospital. Rarely, an abdominal aortic aneurysm ruptures into the inferior vena cava or one of the iliac veins, producing an aortocaval (or aortoiliac) fistula, or it ruptures into the gastrointestinal tract, producing a primary aortoenteric fistula.
The classic clinical manifestations of ruptured aortic aneurysm consist of severe mid or diffuse abdominal pain, shock, and a palpable, pulsatile abdominal mass. The pain may be more prominent in the back or flank, or it may radiate into the groin or thigh. Because the most frequent site of rupture is the left posterolateral wall, pain is more commonly felt on the left side. The pain tends to be severe and steady. The severity of the shock varies from mild to profound, depending on the amount of blood loss. Abdominal distention is common, often preventing palpation of the expected pulsatile abdominal mass. The duration of symptoms can vary from a few minutes to more than 24 hours. Although aneurysm rupture is usually an acute catastrophic event, it can be contained for prolonged periods. These chronic ruptures have masqueraded as radicular compression, symptomatic inguinal hernia, femoral neuropathy, and even obstructive jaundice. It is thought that chronic contained ruptures eventually progress to free ruptures, and they should be treated surgically on an urgent basis.61
Diagnostic Methods
Aortic aneurysms lie against the thoracolumbar spine and project anteriorly in the midline in the epigastrium. Elongated tortuous aneurysms may be located to the right or left or even in the lower quadrants. Careful physical examination can detect most large aneurysms. Except in thin patients, an abdominal aortic aneurysm must be approximately 5 cm in diameter to be detectable on a routine physical examination. As a result, aneurysms are seldom palpated in obese patients unless they are large. The reported accuracy in establishing the correct diagnosis by physical examination alone ranges from 30% to 90%, depending on aneurysm size and body habitus.62 However, even when an aneurysm is palpable, determination of its size by palpation is imprecise. Obesity, ascites, and lack of patient cooperation can impair aneurysmal detection by physical examination. Conversely, tumors or cystic lesions adjacent to the aorta, unusual aortic tortuosity, and excessive lumbar lordosis can all lead to a diagnosis of abdominal aortic aneurysm when none is present. The expansile nature of a pulsatile mass is a key element in deciding whether it is an aneurysm or a transmitted pulsation from an adjacent tumor.
Although physical examination detects most large aneurysms, more objective methods are necessary to measure size and identify smaller aneurysms. Size determination is especially important because it is the most important predictor of rupture risk and is usually the basis of management decisions. Plain abdominal and lateral spine radiographs can establish the diagnosis of 67% to 75% of abdominal aortic aneurysms by detecting linear calcification of the aortic wall (see Figure 39-1). Unfortunately, accurate determination of maximal aortic size is possible in only two thirds of these cases.
Imaging Modalities
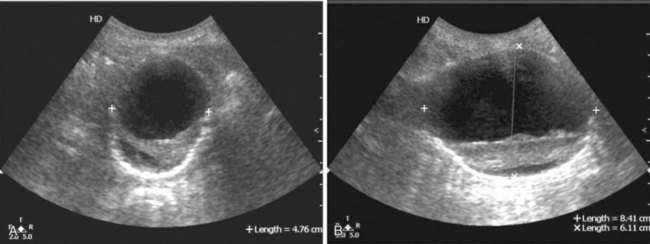
Real-time B-mode ultrasound is available in most hospitals and clinics and is the imaging method used in the large aneurysm screening and surveillance studies.63–65 It uses no ionizing radiation, provides physiologic data as well as structural detail of vessel walls and atherosclerotic plaques, and can accurately measure aneurysm size in longitudinal and cross-sectional directions (i.e., it is three dimensional; see Figure 39-2). Compared with intraoperative measurements, ultrasonic measurements are accurate to within ±5 mm. Many studies have documented the ability of ultrasound to establish the diagnosis (100% sensitivity, 95% to 99% specificity) and accurately determine the size of abdominal and peripheral aneurysms.62–64 Ultrasound has not been as useful for imaging the thoracic or suprarenal aorta because of the overlying air-containing lung and viscera. Similarly, it has been less reliable in defining the relationship between abdominal aortic aneurysms and the renal arteries. Because ultrasonography can obtain images in longitudinal, transverse, and oblique projections, it can be especially helpful in differentiating a tortuous aorta from an aneurysm. Ultrasound imaging is degraded by obesity, intestinal gas, or barium in the bowel. The overlying bowel gas also interferes with evaluation of the iliac arteries. The major advantages of ultrasound are its wide availability, painlessness, absence of known side effects, lack of ionizing radiation, relatively low cost, and ability to image vessels in multiple planes. These factors make ultrasonography the modality of choice for the initial evaluation of pulsatile abdominal or peripheral masses and for follow-up surveillance of aneurysms to determine increase in size and for screening. In addition, the portability of ultrasound machines is advantageous for the emergency department, where it can quickly establish the presence of an aneurysm in most cases, but it is not nearly as accurate (approximately 50%) in demonstrating rupture.
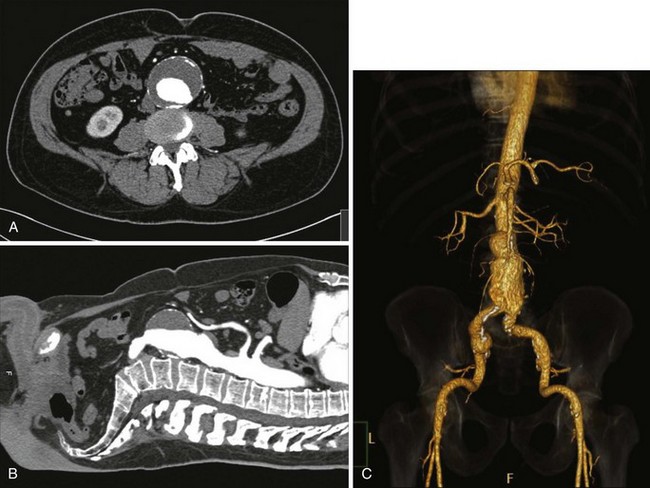
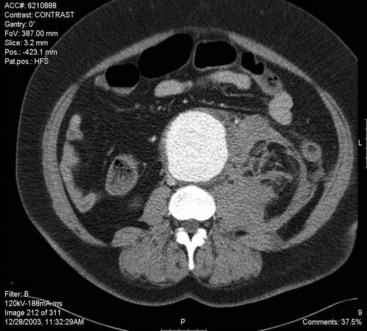
CT uses ionizing radiation to obtain cross-sectional images of the aorta and other body structures. These images provide detailed information about the size of the entire aorta, including the thoracic portion, so that the extent and size of an aneurysm can be measured accurately. Modern CT scanners using helical (spiral) technology and an increasing number of detectors possess sufficient spatial resolution to allow precise identification of the celiac, superior mesenteric, renal, and iliac arteries and their branches, as well as their relationship to the aneurysm and adjacent organs. Major venous structures, including anomalies, can also be identified.49 The administration of intravenous contrast allows evaluation of the size of the aortic lumen, the location and status of aortic branch vessels, the amount and location of mural thrombus, and, in the presence of dissection, differentiation of the true lumen from the false lumen (see Figure 39-3; see color plate for Figure 39-3C). Contrast-enhanced CT scans are also useful for assessing the retroperitoneum and can identify retroperitoneal hematoma (aneurysmal rupture), renal abnormalities, and the periaortic fibrosis associated with inflammatory aneurysms50–53 (Figure 39-4). Metallic surgical clips and orthopedic hardware create artifacts that can interfere with CT interpretation. And although the images are acquired in only one (transverse) plane, three-dimensional reconstructions creating a CT angiogram are now almost universally available. CT provides more information about other abdominal and retroperitoneal structures than does ultrasound. CT scanning has emerged as an excellent technique to image the abdominal aorta and its branches.54 One of its most helpful aspects is the ability to define the relationship of an aneurysm to the renal artery origins. The multiplaner reconstructions are extremely helpful in clarifying the true anatomic relationships when there is anterior or lateral displacement of the aorta.55 Scan times are extremely short, and slices as thin as 2 to 3 mm can be obtained; this allows three-dimensional reconstruction of the overlapping cross-sectional images, producing a CT angiogram (See Figure 39-3C, see color plate). The reconstructed three-dimensional images can be rotated in space and viewed from any projection. CT angiography (CTA) is an excellent method of determining the often complex relationships among the aorta, its branches, and the aneurysm.68,69 CT scans require significant radiation exposure and a relatively large volume of intravenously administered contrast material, which limits their usefulness in the presence of severe renal functional impairment. Non–contrast-enhanced images are useful for determining the degree and location of calcification in the aorta and its branch vessels. Overall, CT scans are currently the most useful imaging method for evaluating the abdominal aorta and have nearly obviated the need for catheter aortography in the evaluation of aneurysmal disease. CT is also an almost essential component of the process of determining an aneurysm’s suitability for and size of endograft treatment.
MRI has also proved to be a useful modality for the evaluation of aortic disease (Figure 39-5).71 MRI uses pulsed radiofrequency energy in a strong magnetic field to produce images in longitudinal, transverse, and coronal planes. MRI instruments are not as widely available as ultrasound or CT scanners, and the selection and interpretation of proper scan sequences and images require considerable experience and skill. The spatial resolution has significantly improved, but the presence of implanted metal-containing devices (surgical clips, cardiac pacemakers) or the need for monitoring equipment are contraindications to MRI. Also, MRI studies are more expensive and require more time than either CT or ultrasound. Nevertheless, MRI clearly distinguishes arteries and veins from viscera and other surrounding tissue, and there is excellent agreement between MRI and ultrasound or CT images in determining aortic diameter. MRI is better than ultrasound for demonstrating involvement of branch vessels, especially the renal arteries, with some authors reporting visualization of the renal arteries in more than 90% of cases.72 Other advantages of MRI over CT are the lack of ionizing radiation, the ability to obtain multiplane images (now also available with CTA), and the relatively large image field. In addition, MRI does not require the use of nephrotoxic contrast agents to achieve intravascular enhancement; however, paramagnetic contrast agents, such as gadolinium, are routinely used to improve the imaging of vascular structures. MRI machines are able to quantitate blood flow, although this feature is not commonly used clinically, and they can produce multiplanar reconstruction of the images that look like conventional angiograms (magnetic resonance angiography [MRA]). However, adequate visualization of aortic branch arteries is not achieved as frequently with MRA as with CTA, and a significant number of patients cannot undergo MR scanning because of claustrophobia.
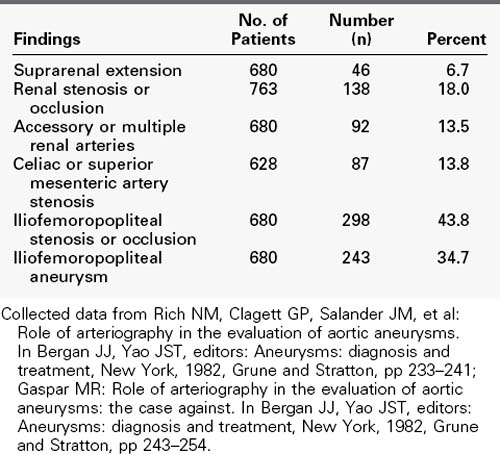
Objective documentation of the aortic size should be accomplished with one of these imaging modalities in all patients with suspected abdominal aortic aneurysms. Each method can measure the diameter accurately. An initial scan can be used for comparison with subsequent scans to monitor aneurysmal enlargement. For most routine situations, ultrasonography is the method of choice because of its widespread availability, lower cost, and lack of ionizing radiation.70 When there is suspicion of suprarenal or thoracoabdominal aortic involvement or dissection, MRI or CT is preferable. For preoperative planning in these complex cases, a multiplanar study with three-dimensional reconstruction (CTA, MRA) is usually necessary to clearly delineate the aneurysm, aortic branch vessels, and neighboring structures. This is especially true if treatment by endografting is being considered. CT and MRI probably have equal capability to demonstrate unexpected features such as venous anomalies, perianeurysmal fibrosis, and horseshoe kidney, although the ureters are not easily identified by MRI. For symptomatic aneurysms, MRI and CT also are better than ultrasound in their ability to identify contained rupture. The limitations of catheter aortography for the diagnosis and evaluation of aortic aneurysms, like those of plain film radiography, are well known. Because the mural thrombus that which is nearly always present tends to reduce the aneurysmal lumen size toward normal, aortography is not a reliable method to determine the diameter of an aneurysm or even to establish its presence. With better imaging methods now routinely available, aortography has little use as a diagnostic method for abdominal aortic aneurysms. However, it may be useful in the preoperative evaluation of selected patients with aneurysms to define the extent of the aneurysm, especially suprarenal and iliac involvement, the associated arterial lesions involving renal and visceral vessels, and distal occlusive lesions (Table 39-3). Although many of these associated lesions are readily detectable and appropriately managed intraoperatively, preoperative identification is useful in planning operative strategy, especially if complex anatomy is suspected. However, in most cases the same information can be obtained with CTA.
Risk of Aneurysm Rupture
As noted earlier, the majority of abdominal aortic aneurysms are discovered in asymptomatic patients during an evaluation for an unrelated problem. Aneurysms are being discovered at a smaller size than when the original studies on their natural history were first published by Estes, Wright, Szilagyi, and others.73–75 Most aneurysms detected in screening programs are small (<4 cm), which has permitted the development of new concepts about the natural history of these lesions. Although aneurysms can cause symptoms and serious consequences from thrombosis and distal embolization, rupture is the most important risk, and aneurysm diameter is the most important factor that determines the risk of rupture. In general, the risk of rupture correlates directly with size: the larger the aneurysm, the greater the risk of rupture. For example, the yearly risk of rupture for abdominal aortic aneurysms5,30,63–65 between 4 and 5.4 cm in size is 0.5% to 1%; this increases to 6% to 10% for aneurysms between 6 and 7 cm and to 19% to 35% for aneurysms larger than 7 cm in diameter.76–78 Calculated as 5-year rupture rates, these figures become 5%, 50%, and about 95%, respectively. The steepness of the curve plotting these data increases sharply at a diameter of approximately 5 cm, which is the basis for recommendations to defer elective aneurysm repair until a size of 5.0 to 5.5 cm is reached.79 These data were provided from two randomized, controlled prospective clinical trials that studied survival in patients with asymptomatic abdominal aortic aneurysms that were between 4 and 5.4 to 5.5 cm in diameter: the United Kingdom Small Aneurysm Trial, published in 1998, and the Veterans Administration–sponsored Aneurysm Detection and Management (ADAM) trial.80–83 These two trials were similar in design, size, and results. The rupture rate for aneurysms in these trials was 0.5% to 1% per year, as noted previously, and neither trial showed a difference in long-term survival, which was the primary end point, between patients allocated to early operation or ultrasonography or CT surveillance. The 6-year survival was similar in both trials, 64% in the U.K. trial and approximately 70% in the ADAM trial. Although 61% of those randomized to surveillance in both studies ultimately underwent aneurysm operation for enlargement or symptoms, long-term survival was not improved by early operation. Furthermore, delaying operation for these small aneurysms was not associated with increased operative or late mortality. These data are consistent with those from older, nonrandomized studies.82,84,85 For example, population-based data from Rochester, Minnesota, showed a mean enlargement rate for aneurysms less than 5 cm in diameter of only 0.32 cm/year, and after 5 years of observation, no aneurysm smaller than 5 cm had ruptured.85 Rupture risk may be higher in high-surgical-risk patients, but the ADAM and U.K. trials enrolled mostly good-risk patients. In both series, there was a higher rupture rate among the ineligible (i.e., high-risk) patients than in those randomized.
Other clinical features associated with increased risk of rupture include smoking, chronic obstructive lung disease, hypertension, female gender, transplant recipient, and rapid enlargement (defined as 1 cm/yr or more). Cronenwett and associates86 showed that chronic obstructive pulmonary disease and systolic hypertension are predictors of increased risk of rupture of small abdominal aneurysms. In a subsequent study, they found that the rate of enlargement of small aneurysms was unpredictable, but either increased systolic or decreased diastolic pressure (i.e., increased pulse pressure) was associated with an increased rate of aneurysm expansion.87 In this study, there was considerable variability in the rate of aneurysm enlargement, although the average rate of expansion was 0.4 cm/yr in anteroposterior dimensions and 0.5 cm/yr in lateral dimensions. Some data suggest that the expansion rate of small aneurysms can be diminished by β-adrenergic blockade (propranolol), which should lead to a decreased rate of rupture.88 Unfortunately, this theory was not supported in three randomized clinical trials. Other studies have shown that aneurysms are frequently elliptical rather than round and that aneurysmal expansion is initially more rapid in the lateral direction. It is interesting to recall that the most frequent site of aneurysm rupture is in the lateral wall. In a review of four series, including their own, Cronenwett and coworkers86 described the outcome of 378 patients with small aortic aneurysms initially treated nonoperatively. After an average follow-up of 31 months, 27% of the patients were alive with intact aneurysms, 29% had died of other causes, 39% had elective aneurysm operations because the aneurysm diameter reached 5 to 6 cm, and 4% had suffered aneurysm rupture or acute expansion leading to emergency operation. Overall, there was a mean 5-year survival of 54% in these patients, somewhat less than the 6-year survival rates of 64% in the U.K. trial and 70% in the ADAM trial.83,84 In light of these more recent studies, autopsy studies showing high rates of rupture of small aneurysms must be interpreted with caution.7 Some have shown that 23.4% of aneurysms between 4.1 and 5 cm rupture, and the same is true for up to 10% of aneurysms less than 4 cm in diameter. Data such as these have led surgeons to recommend operation for almost all aortic aneurysms in good-risk patients. However, autopsy studies underestimate aneurysm size owing to the lack of a distending blood pressure, and several more recent studies on living patients demonstrate a rupture rate of approximately 1% per year for aneurysms less than 5 cm in diameter.
There is little debate about the appropriateness of elective aneurysm surgery for patients with large aneurysms (>5.5 cm in diameter) because of the high risk of rupture and the associated mortality when rupture occurs. This is also true in so-called high-risk patients, because most of these patients will die from rupture and not from the conditions that caused them to be considered high risk. Several cohort studies indicate that women have a greater risk of aneurysm rupture at a given size than men; therefore it has been suggested that women be offered definitive treatment at a smaller aneurysm size (i.e., 4.5 to 5.2 cm) than men.89
It must be emphasized that although the risk of aneurysm rupture correlates most closely with aneurysm size, and the average rate of aneurysmal enlargement is known (0.4 to 0.5 cm/yr), it is impossible to predict when a small aneurysm will rupture in a given patient.74 Perhaps the best explanation relates to the fact that aneurysms rupture at points of maximal wall stress, as discussed earlier, and areas of maximal wall stress do not necessarily coincide with areas of maximal diameter.75,76 Clinical investigations of aneurysm wall stress using finite element analysis of CT-derived data are promising but not widely available. For now, diameter remains the best available predictor of risk of aneurysm rupture. The harmlessness of a small, asymptomatic abdominal aortic aneurysm is deceptive. Some do rupture, but coronary artery disease, and not rupture, is the most frequent cause of death in patients with small aneurysms.
Risks of Surgical Treatment
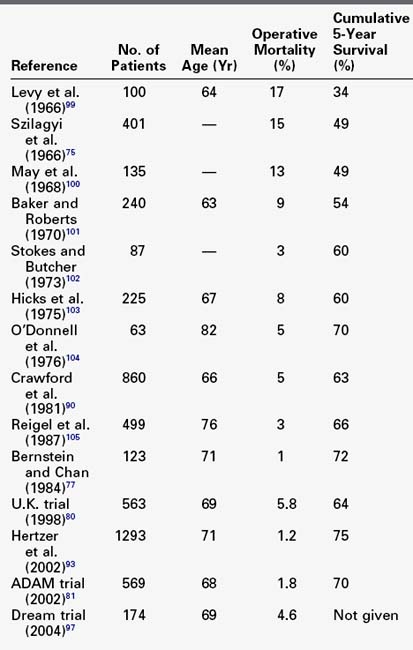
The natural history of untreated abdominal aortic aneurysms is well documented. This is especially true for those aneurysms measuring 5.5 cm or less in diameter. Since the first report of successful surgical resection and graft replacement of an infrarenal aortic aneurysm in 1952, many publications have documented the operative and long-term survival after surgical treatment. There has been a steady improvement in operative results for elective operations. Several large, contemporary series have reported operative mortality rates between 0.9% and 5% for university and other large, single medical centers and only somewhat higher rates for community hospitals.90–106 The 1.8% operative mortality in the ADAM trial from selected VA medical centers compares favorably with these (Table 39-4). Higher mortality rates, ranging from 5% to 8%, have consistently been reported from analyses of large state-wide or national data bases. Lower operative mortality has also been found to be associated with operations performed in high-volume institutions and by high-volume surgeons, although the definitions of high volume are not uniform. The recently published SVS guidelines recommend that elective open surgical repair is best performed at centers with a documented in-hospital mortality of less than 5%. Operative mortality in this range (<5%) justifies elective repair, even for relatively small aneurysms in good-risk patients.
TABLE 39-4 Operative Mortality and Late Survival of Elective Open Surgical Treatment of Abdominal Aortic Aneurysm
Most of the deaths, even in elective operations, occur in so-called high-risk patients. Unfortunately, there is again no consensus on what constitutes high risk. Chronologic age is not as important as physiologic age in assessing operative risk; therefore patients should not be denied elective operation based solely on age. Even octogenarians can undergo elective aneurysm surgery with low morbidity and mortality rates.94,95 Most vascular surgeons have successfully treated ruptured aneurysms in patients previously rejected for elective operation because they were considered too old.
The major risks for elective abdominal aortic aneurysm resection are similar to those for other major intraabdominal operations and include adequacy of cardiopulmonary and renal function. High-risk patients are those with unstable angina or angina at rest, cardiac ejection fraction less than 25% to 30%, congestive heart failure, serum creatinine level greater than 3 mg/dL, and pulmonary disease manifested by room air Po2 of less than 50 mm Hg, elevated Pco2, or both. A substantial percentage of these high-risk patients will die of ruptured aneurysm and not from the disease that led to their categorization as high risk. With intensive perioperative monitoring and support, aneurysm resection has been performed even in these high-risk patients, with operative mortality of less than 6% by Hollier and colleagues107 and others.90,102,103 Therefore, even in high-risk patients, large abdominal aneurysms should be considered for elective treatment if the appropriate support facilities are available. It is in this group, however, that endovascular repair can be expected to offer significant improvement in survival over standard open aneurysm repair. Already in the United States the majority of aneurysm repairs are being treated with this technology; this has resulted in a higher proportion of open repairs being complex, but the anticipated increased morbidity and mortality has not been documented. However, this trend in complexity of open repair could be reversed with more wide-spread use of fenestrated and branched endografts for juxtarenal, pararenal, and suprarenal aneurysms. The consistent findings from clinical trials are that 50% to 60% of patients with abdominal aortic aneurysms are anatomically suitable for endoluminal repair with current commercially available devices and that it can be accomplished with mortality rates equal to or lower than those for open surgical repair. Morbidity rates are clearly lower than those for open surgical repair, hospital stay is shortened, and patient satisfaction and surgeon enthusiasm are high; however, all aortic aneurysms can be treated by open repair. In addition, because of lingering uncertainties about long-term results of endovascular repair, there is uncertainty regarding which patients and which aneurysms are best treated in this manner. For these reasons, the indications for endoluminal repair should be the same as those for open surgical repair in terms of aneurysm size and expected longevity. Endovascular aneurysm repair is covered in detail in Chapters 40 and 41.
The operative results for ruptured aneurysms are not nearly as favorable as those for elective aneurysm repair and generally have not improved greatly over the years.108–111 Although there are a few series with better results, nearly 50% of patients die after being operated on for rupture. The nature of the rupture influences the results. Less than 10% of patients presenting in shock with free intraperitoneal rupture survive. In contrast, patients in stable condition with small, contained leaks have a better than 80% survival rate. Several authors have reported improved mortality rates (30% to 35%) using endovascular treatment for ruptures, and others have reported no survival advantage.112,113 The operative mortality at University Hospital Case Medical Center for open repair of ruptured aneurysms over the past 5 years is 27% (unpublished data), which is in the same range as most of the endovascular repair (EVAR) studies. In one study, endovascular treatment of ruptured aneurysms yielded worse outcomes in nonteaching and low-volume EVAR hospitals.112
The factors contributing to failure in the treatment of ruptured abdominal aortic aneurysm have been reviewed by Hiatt and associates.108 The four most important factors were failure to perform elective aneurysmectomy in patients with known aneurysms; errors in diagnosing rupture when it occurred, leading to delay in operation; technical errors committed during the operation (all venous injuries); and undue delays in induction of anesthesia. These are all realistically preventable. Other series have also attempted to identify factors leading to death after aortic aneurysm rupture. Repeatedly, delays in performing surgery and the total volume of blood transfused are found to be important.109,110 Preoperative cardiac arrest, female gender, age 80 years or older, massive blood loss, and ongoing major transfusion requirements were predictors of 90% to 100% mortality in the series by Johansen and colleagues,111 which included a highly efficient transport and resuscitation response. Some of the differences in operative mortality among various reports are due in part to inconsistencies in patient categorization or considering all forms of rupture together. Many of these series also fail to separate patients with unruptured but symptomatic aneurysms who undergo emergency operations. The operative morbidity and mortality for this group are intermediate between elective, asymptomatic patients and those with frank rupture, averaging 16% to 19%.85,114 It has been postulated that the reasons for this increased mortality is the omission of the usual preoperative evaluation and preparation necessitated by the emergency operation as well as institution-based issues related to emergency procedures during nights and weekends.