Fig. 2.1
Properly positioned left double-lumen endobronchial tube is confirmed by a fiberoptic bronchoscope
OLV is initiated prior to insertion of trocar to prevent lung injury. Respiratory changes may occur during the OLV and the CO2 insufflation. Firstly, ventilation-perfusion relationship may be altered by the modified lateral decubitus position in the anesthetized patient during two-lung ventilation. Perfusion is normally distributed preferentially to dependent regions of the lung because of the hydrostatic gradient imposed by gravity to the benefit of the overall ventilation to perfusion ratio (V/Q) maintained. Nevertheless, general anesthesia, neuromuscular blockade, and mechanical ventilation increase V/Q mismatch. Secondly, intrapleural CO2 insufflation positive pressure constricts the lung, reducing compliance, functional residual capacity (FRC) and tidal volume. OLV may cause further damage to ventilatory conditions by limiting the ventilation of the nondependent lung and create an obligatory right-to-left transpulmonary shunt through the nondependent lung [5, 15, 16]. Last, hypercapnia may occur during OLV and pneumothorax, partly due to CO2 absorption across the pleura, and partly because of a mechanical constriction on the pulmonary parenchyma, reducing tidal volume, FRC, total lung capacity and pulmonary compliance [6, 17, 18]. Because of the above changes, it is not uncommon for a patient to desaturate during OLV. If this happens, the first thing needed is to give the patient a larger breath by hand with sustained pressure at the end of the breath, or applying positive end expiratory pressure (PEEP) to the ventilated lung. That may help to prevent the small airways from closing. Usually 5 cm H2O is enough to keep the saturation up without pushing the peak inspiratory pressure too high (above 40 cm H2O). In fact, if PEEP is too high, blood is diverted to the collapsed lung and the shunt is increased, which will worsen the hypoxemia. Meanwhile, it needs to sure that the patient’s blood pressure has not dropped because dropping of blood pressure may also be the cause of desaturation.
If severe hypoxemia occurs or airway pressure increases significantly, double-lumen endotracheal tube position should be checked at once with fiberoptic bronchoscope to ensure its proper placement. Add continuous positive airway pressure (CPAP 5–10 cm H2O, 5 L/min) to collapsed lung may help to reduce shunt, divert blood to the ventilated lung effectively and to improve oxygenation (Fig. 2.2). When necessary, reinflating the collapsed lung to return to two-lung ventilation temporarily is always an option. Medication to decreased intrapulmonary shunt due to the collapsed lung include using almitrine (12 μg/kg/min for 10 min followed by 4 μg/kg/min) [19] and inhaled nitric oxide that enhance hypoxic pulmonary vasoconstriction (HPV) [20].
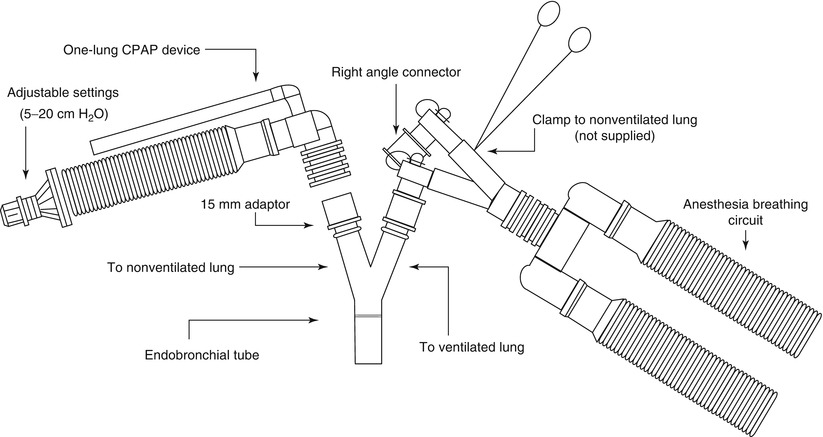

Fig. 2.2
Application of CPAP to the non-ventilated lung
It must be noted that pressure of 5–10 cm H2O will not immediately inflate an already collapsed and atelectatic lung and it may not be very helpful to increase oxygenation right away. Therefore, it is necessary to reinflate the lung with higher airway pressure and then use CPAP to keep lung at a constant level of inflation. High frequency jet ventilation is another method and keeps the lung almost immobile helping with operating conditions [21].
Currently, the definition of permissive hypercapnia has been approved by more and more clinicians. The literature generally agrees that CO2 should be allowed to rise while reducing tidal volume and minute ventilation in order to prevent alveolar overdistention or the propagation of lung injury [22, 23]. The use of excessive airway pressures may increase pulmonary vascular resistance in the dependent lung and increase flow through the nondependent lung. Therefore, the lungs should be protected against high airway pressures during OLV with intrapleural CO2 insufflation, by carefully balancing tidal volume, respiratory rate, minute ventilation and PETCO2 [17, 23–26]. In clinical conditions, PETCO2 that shows the tendency to increase during the course of the OLV and CO2 pneumothorax and sometimes is higher than the normal range, is nevertheless always within the ranges foreseen for moderate permissive hypercapnia [22].
During OLV, arterial oxygenation may markedly decrease because of an increased intrapulmonary shunt due to the collapsed lung. Therefore, it is necessary to increase FiO2 to over 0.5 during OLV and CO2 pneumothorax. The increase of FiO2 is the principal measure allowing an adequate oxygenation. Hypoxic pulmonary vasoconstriction (HPV), a physiologic defense mechanism that serves to divert blood away from poorly ventilated lung regions, is a second way to maintain oxygenation during OLV by restricting pulmonary blood flow to the nondependent lung. OLV management should be reduced to the minimum in any clinical conditions that might directly vasodilate hypoxically constricted lung vessels, such as the presence of infection, vasodilator drug infusion and the use of certain anesthetics (Table 2.1) [12, 27, 28]. Intravenous anesthesia does not influence HPV, while isoflurane, desflurane and sevoflurane have been shown to have less effect on HPV than halothane. To limit their effects on oxygenation, inhalation agents should be used at minimal concentration or less [17, 25]. Also, the intraoperative hypoxia can be reduced by delivering low-flow oxygen to the nondependent lung through a double-lumen bronchial tube or a bronchial blocker with a distal port [15, 27].
Table 2.1
Anesthesiology interventions that modify HPV (Reproduced with permission from Nagendran et al. [28])
Decrease HPV |
Dextran volume replacement |
Halothane |
Halothane and verapamil |
Nifedipine |
Alkalosis (respiratory or metabolic) |
Nitroprusside |
Hypothermia |
Pregnancy |
Increase HPV |
Lateral decubitus position |
Thoracic epidural anesthesia |
Propofol |
Lidocaine |
High-frequency positive pressure ventilation |
Neutral to HPV |
Propofol |
Enflurane |
Isoflurane |
Fentanyl |
CO2 insufflation causes significant circulatory perturbations, alone or in combination with OLV. Cardiovascular function is impaired proportionally to intrathoracic CO2 pressure [29]. An excessive positive pressure results in significant hemodynamic compromise which reduces preload, stroke volume, cardiac index, mean arterial pressure and reflex tachycardia [16, 30, 31]. Therefore, in early stage, the intrapleural pressure is monitored directly via an 18-gauge IV cannula and pressure transducer to avoid excessive pressure generation [10]. Nevertheless, slow increase of the intrathoracic CO2 pressure, limitation of CO2 insufflating pressure, and cardiovascular function optimization (by fluid administration or possible use of inotropic agents) are effective to minimize the hemodynamic changes with only a slight reduction in the preload, but without any major effects on cardiac output and mean arterial pressures [25, 30, 32]. So the hemodynamic compromise is generally well tolerated by the patients with normal cardiac function. A correct way is that the intrapleural insufflation is always performed in stages and the desired intrathoracic pressure should never be attained in less than 1 min.
A recent study on adult thoracoscopy compares OLV with intrapleural CO2 insufflation in respect of the hemodynamic and respiratory effects. The investigation shows a reduction in cardiac index due to intrapleural insufflation that is statistically greater than that provoked by OLV, while the variations in the oxygenation index are statistically greater during OLV with respect to intrapleural insufflation [33]. In one word, intrapleural CO2 insufflation brings about hemodynamic changes characterized by a fall in systolic and diastolic blood pressure greater than in OLV. On the other hand, respiratory endangerment arises due to a greater rise in PETCO2 in patients undergoing OLV than in those where more intrapleural insufflation is applied [34].
In patients with preoperative pulmonary abnormalities, PETCO2 is statistically higher both during OLV and after pleural cavity deflation. The restrictive, obstructive and mixed ventilatory defects need careful ventilatory strategies. In such cases high intraoperative airway pressure should be avoided and a small increase in PETCO2 is preferable [35].
2.5 Induction and Maintenance of Anesthesia
The hemodynamics stability should be taken into consideration in cardiac anesthetic selection (Table 2.2). Today cardiac anesthesia is still based on fentanyl or sufentanil, in combination with etomidate, midazolam, and muscle relaxats for induction and trachea intubation with a double-lumen endotracheal tube. Longer acting muscle relaxants i.e. pancuronium or pipecuronium may subsequently be supplemented to ensure patient paralysis after port placement [36, 37], because patient movement with robotic arms in situ may be devastating [38].
Diazepam | Droperidol | Etomidatea | Ketamine | Lorazepam | Midazolam | Propofol | |
|---|---|---|---|---|---|---|---|
HR | −9 ± 13 % | Unchanged | −5 ± 10 % | 0–59 % | Unchanged | −14 ± 12 % | −10 ± 10 % |
MBP | 0–19 % | 0–10 % | 0–17 % | 0 ± 40 % | −7–20 % | −12–26 % | −10–40 % |
SVR | −22 ± 13 % | −5–15 % | −10 ± 14 % | 0 ± 33 % | −10–35 % | 0–20 % | −15–25 % |
PAP | 0–10 % | Unchanged | −9 ± 8 % | +44 ± 47 % | – | Unchanged | 0–10 % |
PVR | 0–19 % | Unchanged | −18 ± 6 % | 0 ± 33 % | Unchanged | Unchanged | 0–10 % |
PAO | Unchanged | +25 ± 50 % | Unchanged | Unchanged | – | 0–25 % | Unchanged |
RAP | Unchanged | Unchanged | Unchanged | +15 ± 33 % | Unchanged | Unchanged | 0–10 % |
CI | Unchanged | Unchanged | −20 ± 14 % | 0 ± 42 % | 0 ± 16 % | 0–25 % | −10–30 % |
SV | 0 − 8 % | 0–10 % | 0–20 % | 0–21 % | Unchanged | 0–18 % | −10–25 % |
LVSWI | 0–36 % | Unchanged | 0–33 % | 0 ± 27 % | – | −28–42 % | −10–20 % |
dP/dt | Unchanged | – | 0–18 % | Unchanged | – | 0–12 % | Decreased |
There is no single strategy to be recommended for maintenance of anesthesia; hypnotics (midazolam or propofol), opioids and volatile anesthetics are used in different combinations. The dose and selection of anesthetic agents must provide adequate anesthesia and analgesia during induction and surgery, and attenuate the hemodynamic responses to laryngoscopy and surgery. For robotic cardiac surgery, dosages and the type of the drugs that are to be used depend on the desire of ‘fast-track’ – extubation in the operating room or, more commonly, several hours after arrival in the ICU (Table 2.3).
Drug | Induction dose |
|---|---|
Hypnotics | |
Propofol | 1–2 mg/kg |
Thiopental | 2–4 mg/kg |
Etomidate | 0.15–0.3 mg/kg |
Opioids | |
Fentanyl | 3–10 μg/kg |
Sufentanil | 0.1–1 μg/kg |
Remifentanil | 0.1–0.75 μg/kg/min or bolus 0.5–1 μg/kg |
Muscle relaxants | |
Cisatracurium | 70–100 μg/kg |
Vecuronium | 70–100 μg/kg |
Pancuronium | 70–100 μg/kg |
Rocuronium | 0.3–1.2 mg/kg |
Succinylcholine | 1–2 mg/kg |
Maintenance of anesthesia in critically ill patients | |
Sedative/hypnotic agent | |
Propofol | 20–100 μg/kg/min |
Lorazepam | 2–4 mg (25–50 μg/kg) |
Diazepam | 4–8 mg (50–100 μg/kg) |
Midazolam | 0.25–0.5 μg/kg/min |
An opioid infusion | |
Remifentanil | 0.05–0.1 μg/kg/min |
Fentanyl | 0.03–0.1 μg/kg/min |
Sufentanil | 0.01 μg/kg/min |
Dexedetomidine | 0.5–1 μg/kg/h |
High-dose opioids based anesthetic management of the cardiac surgical patients, with more stable hemodynamics providing a long-term mechanical ventilation, has been supplanted by protocols using low-dose fentanyl, sufentanil, or alfentanil [41, 42], to facilitate early extubation. Sufentanil is used in combination with midazolam, propofol and inhaled anesthetics to provide more stable hemodynamics when it is compared with fentanyl-based anesthetic protocols. Sufentanil has a half-life of about 20–40 min and allows patients to awaken within hours of completion of the operation. Remifentanil is a short-acting, esterase-metabolized without any active metabolites, rapidonset μ-opioid receptor agonist, with a context-sensitive half-life of 3–5 min that may be beneficial in shorter operations and in elderly patients [43–45]. It provides stable hemodynamics in high-risk cardiac surgical patients. Remifentanil-propofol combination is usually selected for patients who can be extubated promptly after surgery. Thus, propofol has not been shown to increase overall hospital costs [46].
Midazolam has been shown to have an average elimination half-life of 10.6 h in patients recovering from cardiac surgery [47]. Although early extubation can be achieved in patients receiving midazolam throughout surgery, most groups limit its use to the pre-bypass period. Propofol providing stable hemodynamics at the recommended doses of infusion 2–4 mg/kg/h and continue it in the ICU [4, 8]. Propofol decreases systemic blood pressure and systemic vascular resistance because of its strong vasodilator properties. The major advantage of using propofol is the early extubation leading to shortening the length of stay in ICU. When the patient is stable, propofol is turned off and the patient is allowed to awaken [48].
Cason et al. [49] first described the term anesthetic preconditioning in 1997, by showing protective effect of isoflurane applied shortly before ischemia. Since then numbers of experimental studies revealed the cardioprotective efficacy of volatile anesthetics [50]. Agents commonly used include isoflurane, enflurane, desflurane, and sevoflurane, which are generally given during CPB to maintain anesthesia and reduce blood pressure, and allow to decrease the doses of intravenous medications. Desflurane and sevoflurane have less lipid solubility with a rapid onset of action and are quickly reversible, allowing for early extubation. A recent meta-analysis the choice of desflurane and sevoflurane results in better outcome in terms of mortality and cardiac morbidity in cardiac surgical patients [51]. Although the exact mechanism of preconditioning of volatile anesthetics is not yet known, the cardiac depressant effects that reduces myocardial oxygen demand, were demonstrated to have direct cardioprotective effects [50]. Nitrous oxide is contraindicated because it reduces the amount of oxygen that can be delivered and may also increase pulmonary arterial pressures.
Muscle relaxants are given throughout the operation to minimize patient movement and suppress shivering during hypothermia. Pancuronium offsetting the bradycardia effect of high-dose opioids has been the prefered neuromuscular blocker, however it has also been shown to have potential to produce a tachycardia causing myocardial ischemia during induction. In contrast, vecuronium and pipecuronium have very few hemodynamic effects. Rocuronium is a short-acting neuromuscular blocker with a rapid onset of action and vagolytic properties. It provides more adequate conditions especially for fast track anesthesia due to its less residual blockade and shorter time to extubation [52]. Atracurium does not undergo renal elimination and is the best agent to be used in patient with renal insufficiency [53].
2.6 Anesthetic Technique
In robotic cardiac surgery, anesthesiologist should be versed in cardiac and thoracic anesthesia and familiar with the skills required for TEE and nonsternotomy CPB. Strategies of respiratory management are the key point of anesthesia involving OLV and CO2 pneumothorax. However, OLV and CO2 pneumothorax can be the cause of hemodynamic instability and should be closely monitored. CO2 insufflation in the chest cavity will lead to an increase in peak airway pressure, particularly during OLV. Usually, double lumen tube is mostly used for OLV and should be replaced by a single lumen tube following surgery. However, care must be taken that swelling of the glottis and pharynx resulting from intubation and TEE might make reintubation difficult. In a few cases in which the airway is deemed to be difficult, Univent tube or bronchial blocker should be used, and optimal position is achieved with the use of a fiberoptic bronchoscope [54]. Although they do not allow CPAP or suction to the collapsed lung, they do not need to be replaced at the end of surgery. They may be placed more easily in patients who are difficult to intubate or who have a small glottis opening, which often would not accommodate the large double lumen tube.
TEE guidance of cannula during peripheral cardiopulmonary bypass is crucial to success. Femoral-Femoral bypass is the most commonly used technique. Complications from arterial cannulation are reduced when TEE is used to confirm the location of the arterial wire within the aorta. Venous cannulae must also be guided into the inferior vena cava and into the superior vena cava [55]. Also, the surgeons now rely almost exclusively on high-quality intraoperative TEE imaging to plan mitral valve repair. A saline test is performed intraoperatively to confirm the echocardiographic findings, and occasionally measure the valve segments directly [56].
Because the side cart is close to the patient’s head, there is limited access to the patient’s airway and neck. Anesthesiologist and the bed-side assistant must be guarded against patient-robot collision during surgery, which is defined as a limitation in the free movement of the robot’s telemanipulated arms by interference with the patient’s body [57]. After the robot is engaged, the patient’s body position cannot be changed. The surgical team should be capable of rapidly disengaging the robotic device if an airway or anesthesia emergency arises. An optimal surgical position is that the patients are placed in an incomplete side-up position at a 30° angle right or a left lateral decubitus position. Positioning the arm of the elevated side along the side with gentle elbow flexion has reduced conflict with robotic arms and decreased risks of a brachial plexopathy. It is important to take care of avoidance of unnecessary stretching of the elevated side arm because it can produce damage to the brachial plexus. A recent case report [58] described a brachial plexus injury in an 18-year-old male after robot-assisted thoracoscopic thymectomy. In this report the left upper limb was in slight hyperabduction. In some centers, the arm of the elevated side is hanged above the head. The elevated arm should be protected by using a sling resting device [59].
Before the arm of the robot is in the chest cavity, a complete lung collapse must be maintained throughout the procedure. Robotic surgery with the da Vinci Surgical System does not allow for changes in patient position on the operating table once the robot has been docked. Close communication between the surgeon and anesthesiologist in relation to the positioning and functioning of the robot is mandatory.
As the heart is limited by the minithoracotomy approach, cardiac defibrillation must be provided by means not requiring direct epicardial contact. External defibrillator pads are placed on the back and the opposite chest wall. Successful termination of ventricular fibrillation (VF) by electrical defibrillation is dependent on the delivery of sufficient electrical current through the heart to depolarize a critical mass of myocardial tissue [60–62]. Transmyocardial current is directly related to the energy delivered, and inversely related to transthoracic electrical impedance (TTI) [63]. Because air and CO2 in the chest act as electrical insulators, they can increase both TTI and defibrillation thresholds [64]. There are other proofs, pneumothorax has been linked to repeated failed defibrillation and increased energy requirements during induced VF with implantable cardioverter-defibrillator placement [65–67]. In these cases, resolution of pneumothorax results in improvement in defibrillation thresholds. Hatton et al. [68] reported a case of multiple failed defibrillation attempts during robot-assisted LIMA harvest during OLV that VF might be resulted from inadvertent pericardial application of electrocautery. The 4th time of external defibrillation attempt with resumption of two-lung ventilation and decompression of the iatrogenic pneumothorax, the patient was successfully defibrillated.
The bulk of the robot is positioned over the abdomen and chest. Although the incidence of airway or serious cardiovascular events are no greater in robot-assisted surgery, if they do occur, the position of the robot will interfere with effective cardiopulmonary resuscitation and airway interventions [69]. The theatre team should practice an emergency drill for the removal of the robotic cart.
The successful use of the robot to assist in surgery depends upon excellent communication among all members of the theatre team. The surgeon sits behind a console, away from the site of operation, but must communicate effectively with both anesthetic staff and his operative assistant at the patient’s bedside. The special care must be taken to ensure that transfer of information is precise and clear. This is aided by the addition of audio speakers to the video tower that transmits the operating surgeon’s voice.
The postoperative course is usually uneventful. On arrival in the cardiac surgery ICU, all patients remain sedated (usually with propofol) until hemodynamics become completely stable and with minimal blood drainage. The incidence of complications is low. Blood transfusion is not normally required as intraoperative blood loss is very low, but significant hemorrhage may be insidious and the patient should be carefully monitored in the immediate postoperative period. Postoperative analgesic considerations are similar to those corresponding non-robotic operations.
2.7 Anesthesia Consideration of Robotic Assisted CABG on Beating Heart
For robotic assisted CABG on beating heart, fast-track anesthesia with early extubation appears to be normal, which can involve either extubation immediately at the conclusion of surgery or within a few hours of arrival in the ICU. The goal of minimally invasive coronary artery bypass grafting is to perform the entire anastomosis endoscopically and avoid CPB. The result is to reduce postoperative morbidity, length of hospital stay, and overall cost. Advances and experience in beating heart surgery have aided this approach.
The first closed-chest CABG surgery with the aid of robotic instruments was performed on human in June 1998 [70]. Thereafter, the technique of robotic assisted CABG has achieved perfection. Technical advances in minimally invasive surgery have enabled CABG to be performed through very small ports. Sternotomy alone carries a finite risk of morbidity from an inflammatory response, but it is less than that of exposure to cardiopulmonary bypass [71, 72]. Robotic assisted CABG on beating heart (off-pump) includes two kinds of surgeries: TECAB and MIDCAB in which the left internal mammary artery (LIMA) is harvested robotically and direct anastomosis through a small left anterior thoracotomy incision (6 cm) [73–75]. Although the anesthetic concerns in managing robotic assisted CABG operation are similar to those of any patient requiring surgical revascularization, this surgical technique requires greater communication and coordination between the surgical team and the anesthesiologist. The anesthesiologist in this setting must provide both hemodynamic stability and relative bradycardia. Because the minithoracotomy incision permits only limited access to the heart, the anesthesiologist must give early warning of impending cardiovascular collapse and the need for emergent institution of CPB. So a perfusionist should always be prepared in the operating room in the event of the need for CPB.
Performance of the distal anastomosis involves the use of a myocardial stabilizer designed to isolate a small segment of myocardium with the relevant coronary artery, and proximal and distal silastic sutures to control back-bleeding [10]. Intracoronary shunts are often used to facilitate anastomosis and maintain distal perfusion, particularly to the target vessel that the myocardium perfusion still depends on. But putting the intracoronary shunt into the target vessel is not easy in TECAB surgery and it may disturb the anastomosis with continuous suture or U-clip. Alternatively, it can be done to provide ischemic preconditioning to assess the possibility of not using intracoronary shunt during the anastomosis, a 5 min test occlusion of the LAD is conducted before coronary arteriotomy [76]. If this is well tolerated, the LIMA to LAD anastomosis is completed without CPB. If signs of ventricular dysfunction appear, median sternotomy may be performed, and the patient can be placed on CPB, or alternatively, femoral-femoral CPB may be initiated.
In our institution, the details of anesthesia are as follows [4, 8]. Patients are prepared and draped as for conventional cardiac surgery, permitting sternotomy in case of need. All cardiac medications should be continued up to the day of surgery. Patient monitoring consists of standard electrocardiogram leads II and V5 and a right radial artery catheter placed under local anesthesia before induction. Intubation is performed with a left-sided double-lumen endotracheal tube and correct placement is confirmed by both auscultation and fiberoptic bronchoscopy. After intubation, a pulmonary artery catheter is placed in the right internal jugular vein (RIJV). A two-lumen central venous catheter is also placed in the RIJV in all patients. TEE probe is placed after all central catheters are inserted. This anesthetic protocol permits patients to be entered into an early extubation and fast-track recovery protocol. Postoperative analgesia is administered with intravenous sufentanil.
It is important that throughout the operation the heart rate remains slow and stable. The combined use of nitroglycerin and beta-blockade minimizes the possibility of myocardial ischemia during the period of vascular occlusion. If systemic hypotension occurs as a consequence of the procedure or from the anti-ischemia therapies, small amounts of phenylephrine or norepinephrine are administered to transiently augment vascular tone and restore systemic pressure. The effects of drug administration on the cardiac index, pulmonary artery pressures, and ventricular function as assessed by TEE are closely monitored. Usually, CO2 insufflation and OLV increase central venous pressure and pulmonary artery pressure by a small amount [77].
For the multivessel revascularization indication, both internal thoracic arteries (ITA) may be used for grafting of the LAD, diagonal branch, right circumflex, and right coronary artery. Bilateral ITA grafting is feasible but appears to be very challenging and time consuming. Such a procedure should be accepted only for very special indications [78]. For harvesting both ITA, insufflation of the left hemithorax is sufficient to expose the right internal mammary artery because of the leftward position of the heart and the improved angle of sight. If the right side pleural is broken, both sides of intrapleural CO2 positive pressure may cause further damage to ventilatory conditions by limiting the ventilation of the right lung and increased CO2 absorption across the pleura, and this may result in hypercapnia and tachycardia more easily. To control heart rate slower and stable is difficult in the situation. Most patients studied tolerate bilateral pneumothorax well for periods less than 1 h [79].
Graft patency is usually evaluated intra-operatively using direct measurement of blood flow by means of a Doppler flow meter. The blood flow measured is thus dependent on systemic blood pressure and distal coronary run-off.
2.8 Anesthesia Considerations of Minimally Invasive Mitral Valve Surgery with da Vinci Surgical System
Technological innovations are improving minimally invasive mitral valve surgery as da Vinci Surgical System becomes a feasible, safe and effective option in mitral valve surgery [80, 81]. It is claimed that the robotic mitral valve repair and replacement allow complete anatomic correction of all categories of leaflet prolapses, enhance visualization of the valve which can afford a high mitral valve repair rate, offer excellent freedom from adverse events, decrease ventilation time and length of stay [82, 83], and have excellent early-term and mid-term results [84].
The preoperative considerations required for anesthesiologists is to evaluate and estimate the cardiac and lung functions of the patients, as well as the change of pathophysiology of heart, the tolerance to OLV and CO2 pneumothorax, and the effect of CPB. All these need adapt the prudent strategy to manipulation of the infusion, use of inotropic and reasonable ventilation management in order to maintain the stable of hemodynamic and effective deal with hypoxemia. From the induction of anesthesia to start the CPB, it is crucial to maintain stable hemodynamic and oxygenation. An understanding and an appreciation of pathophysiologic changes associated with mitral stenosis and mitral insufficiency form the foundation of anesthetic management in this kind of patients. With long-standing mitral valve disease, the elevated left atrium pressure leads to passive increases in pulmonary arterial and venous pressure. During surgery the CO2 pneumothorax is needed which may artificially create a positive intrathoracic pressure and results in relative hypovolemia by reduce the venous return. In addition, most patients with valve heart disease have increased dependency and sensitivity to ventricular preload. The adequate intravascular fluid is not only benefit to keep heart rate in an optimal range but also to maintain the cardiac preload. Tachycardia has detrimental effects on mitral stenosis because of the decreased time for diastolic filling. For mitral insufficiency patients, especially with ventricular distention, the inotropic (dopamine or epinephrine) is often used to maintain a stable hemodynamic condition.
Hypoxemia may occur during OLV, especially in the post-CPB phases. The following factors may contribute to Hypoxemia. Firstly, left OLV is more harmful to oxygenation. Slinger et al. [85] found that the side of position to be one of the important factor in predicting hypoxemia during OLV, which may be relevant to the fact that the right lung is larger than the left one. A recently study also found that, while ventilating with an FiO2 of 1, mean arterial oxygen tension was approximately 280 mmHg on the left-sided as compared with approximately 170 mmHg on the right-sides thoracic operation during OLV [86]. Secondly, the airway is narrowed because of the use of double-lumen endotracheal tube, prolonging alveolar emptying time. Some alveoli are over inflated and damaged because of the high inspiratory pressures during OLV resulting in alveolar edema [38]. Fortunately, the hypoxic pulmonary vasoconstriction (HPV) is beneficial to reduce the perfusion of the nonventilation lung and improving oxygenation [87]. It is demonstrated that the level of tidal volume during OLV could be maintained just as that during two-lung ventilation without positive end-expiratory pressure, targeting normalization of CO2 [88, 89]. Thereby, it is reasonable to keep the tidal volume as in two-lung ventilation and regulate the respiratory rate (10–15 bpm) to maintain the PETCO2 in 40 mmHg.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


