Anesthesia for Pediatric General Thoracic Surgery
Narasimhan Jagannathan
Kenneth Langen
Conditions requiring thoracic surgery can affect children of any age. Anesthetic considerations in the older child and adolescent are similar to those in the adult patient. The greatest challenge to the pediatric anesthesiologist is the anesthetic management of the neonate. Variables such as the newborn’s small size as well as the unique anatomic, physiologic, and pharmacologic differences make neonatal anesthesia a field unto itself.
Physiologic Considerations in the Neonate
Cardiovascular Adaptation
The cardiovascular system undergoes several changes during the transition to extrauterine life. Closure of the ductus venosus and foramen ovale converts the circulatory system from a parallel circuit to a series circuit. Hypoxia, hypercarbia, sepsis, and hypothermia can cause undesirable right-to-left shunting of blood through the foramen ovale and ductus arteriosus, the anatomic closure of which may not be complete until 2 weeks after birth. Pulmonary vascular resistance, elevated during fetal life and immediately after birth, decreases rapidly at first and attains adult values by 2 months of age. During this time, however, the pulmonary vascular resistance is labile, and considerable constriction and dilation can result from physiologic, pharmacologic, and environmental manipulations. The syndrome of persistent pulmonary hypertension of the newborn—characterized by refractory hypoxemia, hypercarbia, and acidosis—is common in patients with diaphragmatic hernias but can occur in virtually any stressed term infant.
Because it must work against increased resistance in utero, the right ventricle is hypertrophied and dominant in the newborn. Both the left and right ventricles are noncompliant and myocardial tissue of the newborn has 30% fewer contractile elements than that of the adult.89
Increases in preload cannot increase stroke volume because of the diminished contractility of the newborn myocardium.81 The infant is functioning on an unfavorable portion of Starling’s curve; only a modest increase in filling pressure can precipitate congestive heart failure. Therefore the anesthesiologist must scrupulously avoid overzealous administration of intravenous fluids.
The cardiac output of a newborn (180–240 mL/kg per minute) is two to three times the adult value relative to size. This difference reflects the greater oxygen consumption and metabolic rate in this age group. Increases in cardiac output are achieved primarily by increases in heart rate (normal, 120–160 bpm), because the infant’s myocardial contractility is relatively fixed. Sympathetic innervation of the heart is incomplete, further impairing the ability to increase stroke volume.94 Systemic blood pressure is low in the newborn period (Table 24-1).77 Furthermore, infants with birth asphyxia and those on ventilators have lower blood pressures.40 Awareness of normal values is essential for the appropriate diagnosis and treatment of hypotension.
Respiratory Adaptation
The trachea has an incompletely developed cartilaginous framework. Any extrathoracic obstruction, such as postextubation mucosal edema, can cause tracheal collapse distally. Even a 1-mm ring of tracheal narrowing can cause severe respiratory distress because of the already small airway caliber (Fig. 24-1). The infant has a highly compliant chest wall because of a horizontally oriented rib cage.63 In diseases of poor lung compliance (e.g., pulmonary edema and atelectasis), excessive lung recoil results in greater retraction of the soft chest wall and more loss of functional residual capacity than would occur in older children with stiffer chest walls.
When the newborn is supine, its closing capacity impinges on normal tidal breathing. As a result, small airway collapse leads to atelectasis, ventilation/perfusion mismatch, and hypoxia.73 The anesthesiologist can use controlled ventilation and positive end-expiratory pressure (PEEP) to help prevent this situation.
Because of their high oxygen consumption and increased work of breathing, infants breathe at rapid rates of 30 to 50 bpm. The diaphragm of an infant has a preponderance of fast-twitch muscle fibers, which are prone to early fatigue.19 Conditions causing increased work of breathing are therefore not tolerated for long periods of time and may result in hypercarbia and respiratory failure.
Chemical and neural control of breathing is different in the newborn. The response to hypoxia is paradoxic, characterized by a brief period of hyperpnea, followed by apnea.70 The central
chemoreceptors have a diminished sensitivity to PCO2 compared with those of adults, that is, a higher PCO2 is needed to effect a similar increase in minute ventilation. Periodic breathing and apneic spells are common in the newborn, making close monitoring of respiratory function mandatory in the postoperative period.68,69
chemoreceptors have a diminished sensitivity to PCO2 compared with those of adults, that is, a higher PCO2 is needed to effect a similar increase in minute ventilation. Periodic breathing and apneic spells are common in the newborn, making close monitoring of respiratory function mandatory in the postoperative period.68,69
Table 24-1 Relationship of Age to Normal Blood Pressure (mm Hg) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Oxygen unloading at the tissue level is made more difficult by the high percentage of fetal hemoglobin in newborn erythrocytes. Because of its lower P50, hemoglobin F “holds on” to oxygen more tenaciously than does adult hemoglobin. The generally high hemoglobin concentration at birth (15–18 g/dL) is beneficial in increasing oxygen delivery to the cells.
Metabolic Adaptation
Maintenance of normothermia is essential in the newborn. Adverse effects of hypothermia include apnea, hypoglycemia, metabolic acidosis, and increased oxygen consumption. Because of decreased subcutaneous tissue, a low surface area-to-volume ratio, and small body mass, the neonate has increased environmental heat losses. Nonshivering thermogenesis, mediated by catecholamine effects on brown fat deposits, is the primary heat-generating process in the newborn. This process increases oxygen consumption by as much as 200-fold. Methods of preventing heat loss intraoperatively include increasing ambient temperature and using radiant warmers, heating blankets, intravenous fluid warmers, and humidified anesthetic gases.12 Covering the top of the head and extremities with plastic wrap effectively minimizes evaporative heat losses during surgical procedures.6 Moreover, the use of forced-air warming covers and passive heat- and moisture-exchanging filters has significantly diminished heat loss in pediatric surgical patients.13,53
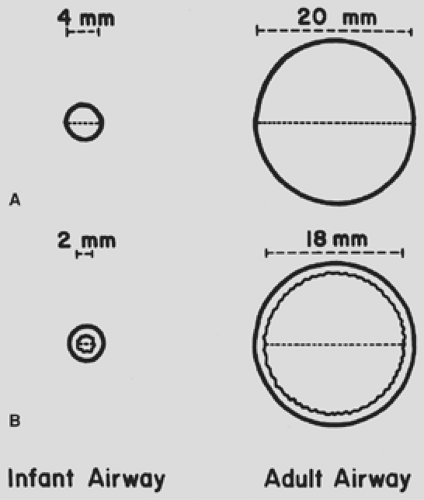 Figure 24-1. Diagram of relative cross-sectional area of infant and adult trachea. A: No tracheal edema. B: One millimeter of edema encircling tracheal lumen. |
Hypoglycemia occurs frequently in this age group, especially in the premature infant, the small-for-gestational-age infant, and the infant of a diabetic mother. Causative factors in the development of neonatal hypoglycemia include diminished hepatic glycogen stores, decreased gluconeogenetic capabilities, and decreased response to glucagon secretion.74 Blood glucose values should be monitored frequently and adjusted using dextrose containing fluids accordingly. Normal blood glucose values in the newborn are listed in Table 24-2.75
Pharmacologic Considerations in the Neonate
Virtually all drugs used in the practice of adult anesthesia have been safely used in pediatric anesthesia. Because of the physiologic characteristics of the newborn, the pharmacokinetics and pharmacodynamics of many commonly used medications are significantly different from those used in the adult population. Drug dosages must frequently be altered from adult values, and target-organ responses must be monitored carefully. Drugs that inhibit hypoxic pulmonary vasoconstriction (HPV) can contribute to ventilation/perfusion mismatch and potential hypoxemia, especially during one-lung anesthesia. The choice of anesthetic agents depends upon the patient’s cardiopulmonary status and the surgical lesion.
Inhalation Agents
Inhalation anesthetic agents are commonly administered with O2 and air during maintenance of anesthesia. Anesthetics such as nitrous oxide, sevoflurane, desflurane, and isoflurane have been used in pediatric thoracic surgical patients. Sevoflurane
has many advantages in the pediatric patient owing to the combination of rapid onset and nonpungency, making this agent ideal for inhalation induction.54 Isoflurane may be preferred for thoracic surgery since it exhibits less attenuation of HPV compared with other inhalation agents. All volatile agents cause a dose-dependent depression of cardiac function in addition to impaired baroreceptor reflexes.18,84 Hypotension and bradycardia commonly occur when potent inhalation agents are administered in high concentrations. Because all potent inhaled anesthetics have a low therapeutic index in infants, they must be used sparingly, with close attention to blood pressure and heart rate. Nitrous oxide can increase pulmonary vascular resistance, which is undesirable in neonates, with their potential for persistent pulmonary hypertension.26 Clinical studies have shown, however, that nitrous oxide can be used in infants without a significant increase in right-to-left shunting.41
has many advantages in the pediatric patient owing to the combination of rapid onset and nonpungency, making this agent ideal for inhalation induction.54 Isoflurane may be preferred for thoracic surgery since it exhibits less attenuation of HPV compared with other inhalation agents. All volatile agents cause a dose-dependent depression of cardiac function in addition to impaired baroreceptor reflexes.18,84 Hypotension and bradycardia commonly occur when potent inhalation agents are administered in high concentrations. Because all potent inhaled anesthetics have a low therapeutic index in infants, they must be used sparingly, with close attention to blood pressure and heart rate. Nitrous oxide can increase pulmonary vascular resistance, which is undesirable in neonates, with their potential for persistent pulmonary hypertension.26 Clinical studies have shown, however, that nitrous oxide can be used in infants without a significant increase in right-to-left shunting.41
Table 24-2 Normal Blood Glucose Values | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Induction Agents
Thiopental is a commonly used induction agent in newborns. Lower doses should be used (2–3 mg/kg) in this population because of the immaturity of the blood–brain barrier and a relatively high cerebral blood flow, resulting in a larger fraction of drug delivery to the brain.91 In addition, unacceptable degrees of hypotension may result from the use of large doses, as thiopental is a myocardial depressant.
Ketamine is a potent amnesic and analgesic agent that can be given intravenously (1 to 2 mg/kg) or intramuscularly (5 mg/kg) for the induction of general anesthesia. Cardiovascular hemodynamics and spontaneous respirations are maintained because of sympathetic nervous system stimulation, accounting for the popularity of this drug in pediatric anesthesia.92 Ketamine causes copious salivation, so prior or concurrent administration of an antisialagogue such as atropine is often necessary.
Etomidate is a useful induction agent when cardiovascular dysfunction or cardiomyopathy is present in children.24 The major advantage of this drug is hemodynamic stability during induction. Nausea, vomiting, and adrenal suppression are disadvantages which limit its usefulness.
Propofol has gained widespread use for induction as well as maintenance of anesthesia in pediatric patients. Its major advantages are a short half-life, rapid recovery, and effective obtundation of airway reflexes.64 HPV is not inhibited by propofol.86 Age-dependent induction doses of 2 to 3 mg/kg were found effective in children.90 For maintenance of anesthesia, propofol is usually administered by infusion. Higher infusion rates are required for children than for adults.57 Dose-dependent decreases in heart rate and mean arterial blood pressure can be seen after propofol administration. Long-term use of propofol for sedation in the intensive care unit has been associated with metabolic acidosis, bradyarrhythmia, heart failure, and death.15,46
Miscellaneous Agents
Paralysis is often required for intrathoracic procedures in order to facilitate surgical exposure, ablate respiratory effort, and decrease the requirement for supplemental anesthesia.60 Muscle relaxants such as the nondepolarizing agents supplement almost all anesthetics used in newborns. Nondepolarizing relaxants such as pancuronium, atracurium, cisatracurium, vecuronium, and rocuronium have been used extensively in pediatric patients.31,60,61 The rapid onset of paralysis along with a shorter duration of action with rocuronium makes this drug preferable for shorter procedures. Succinylcholine is not routinely used in pediatric airway management; however, it is the agent of choice for emergency situations where the airway needs to be secured rapidly, as in laryngospasm.32 Infants require larger doses of succinylcholine, calculated on a per kilogram basis, because an increased extracellular fluid compartment results in a larger volume of distribution.19,33
Fentanyl, a synthetic, short-acting, potent narcotic, has been used extensively in neonatal anesthesia. Its primary advantage is cardiovascular stability.43,80 Attenuation of the pulmonary vasoconstrictive response to tracheal stimulation and lack of histamine release are additional benefits.42 Sufentanil is a more potent narcotic than fentanyl and is commonly used for cardiothoracic procedures. However, sufentanil has no clear-cut benefit over the more familiar, less expensive fentanyl.66
Atropine is used in pediatric anesthesia for its anticholinergic (antivagal) properties. It counteracts the undesirable bradycardia associated with halothane and succinylcholine administration, vagal stimulation during laryngoscopy, and intraoperative visceral traction. Slow heart rates can lead to a decrease in cardiac output, because the neonate cannot compensate by increasing its stroke volume.
Monitoring
The purpose of monitoring any variable during anesthesia is to identify adverse trends before they become catastrophic events. Because of their diminished cardiopulmonary and metabolic reserves, infants require close intraoperative monitoring. Confounding the goal of vigilant invasive and noninvasive monitoring is the infant’s small size, which can make even the simplest procedures, such as applying electrocardiography leads, frustrating. After anesthetic induction with standard monitors, intravenous catheter placement, and tracheal intubation, an arterial catheter is usually inserted in most patients undergoing thoracotomy as well as those with severe lung or cardiac disease. The placement of a central venous catheter should also be considered, depending upon the patient’s comorbidities as well as the type of surgery to be performed.
Standard Monitors
Temperature Probe
Rectal or esophageal temperature most closely approximates core temperature and is preferred over skin and axillary monitoring (Table 24-3).
Electrocardiography
Cardiac rate and rhythm are the primary data obtained from this monitor. Detection of ischemic events is not as important in pediatric patients because coronary artery disease and resulting regional ischemia is uncommon in this age group. For this reason, a three-electrode system is generally used in neonates, infants, and children, as opposed to the five-lead system commonly used to monitor adults. Smaller electrodes are available for placement on the trunk, as well as limb leads
designed for use around the wrists and ankles. When regional anesthesia is being used, monitoring of T-wave changes also provides early detection of inadvertent intravascular injection of local anesthetic agents containing epinephrine.78
designed for use around the wrists and ankles. When regional anesthesia is being used, monitoring of T-wave changes also provides early detection of inadvertent intravascular injection of local anesthetic agents containing epinephrine.78
Table 24-3 Commonly Used Monitors for Pediatric Thoracic Anesthesia | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Blood Pressure Cuff
Automated noninvasive blood pressure monitoring equipment for children has led to the nearly universal use of this technology for accurate, reliable determination of systolic and diastolic pressures. Alternatively, the systolic blood pressure can be measured by looking for the to-and-fro movement of the sphygmomanometer needle.
Oxygen Analyzer
The oxygen analyzer is inserted in the inspiratory limb of the anesthesia circuit. This allows the anesthesiologist to continuously monitor and adjust the inspired oxygen concentration. Prolonged hyperoxia can lead to retinopathy in the infant whose postconceptual age is <46 weeks, in whom the retina has not completely matured.55
Pulse Oximeter
Pulse oximetry is used primarily for the detection of hypoxemia and assessment of oxygenation. The plethysmographic tracing of pulse oximetry saturation also can be used to monitor the circulation.82 For operations associated with a high risk for intraoperative hypoxemia, such as repair of a tracheoesophageal fistula, the use of pulse oximetry has made early detection of impaired oxygenation possible.7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


