Chapter 4 Anatomy and Surgical Exposure of the Vascular System
Exposure of the Carotid Bifurcation
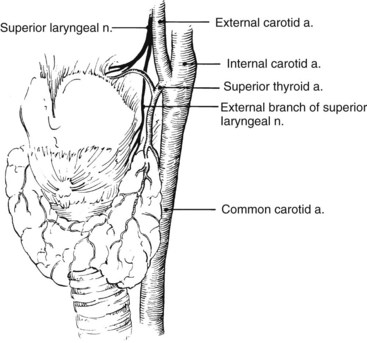
The surgeon must also maintain an awareness of the location of the vagus nerve and its branches. It lies within the carotid sheath between the common carotid artery and the internal jugular vein. Normally, it is directly behind the internal carotid artery at its origin. Care must be taken to prevent injury to the nerve at this vulnerable location. Additional care is required to prevent vagus nerve injury during repeated carotid exposure, because the nerve, which may be encased in scar tissue, frequently courses anterior to the carotid bifurcation. The superior laryngeal nerve arises from the vagus nerve above the carotid bifurcation, passes behind the internal carotid artery, and descends medial to the superior thyroid artery. Care must be taken during mobilization of this vessel not to injure the superior laryngeal nerve or its external branch (Figure 4-1). The external branch of the superior laryngeal nerve sometimes passes between the branches of the superior thyroid artery or is adherent to it. Table 4-1 lists the locations and the tests for function of the important nerves encountered during exposure of the carotid bifurcation.
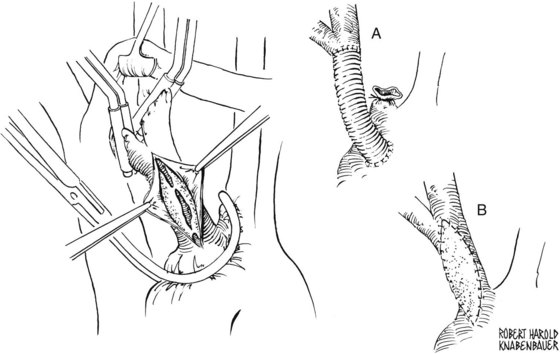
A carotid arteriotomy should be created proximal to the carotid bulb and lateral to the carotid flow divider in the typical endarterectomy scenario. This incision is then lengthened distally through the diseased internal carotid artery under direct vision to a point where there is normal-appearing intima. It is critical not to make this arteriotomy on the anterior aspect of the internal carotid artery near the carotid sinus, because this is a relatively fixed area that is difficult to reapproximate without creating a focal narrowing that is at risk for restenosis. It is wise to find the correct endarterectomy plane at the level of the carotid bulb. Endarterectomy then proceeds proximally first, and the specimen is excised sharply with Potts scissors at the level of the common carotid artery. Everting the external carotid artery into the carotid bulb facilitates endarterectomy at this level. Next, the transition point between the atherosclerotic plaque to be removed and the remaining nondiseased internal carotid artery is located. This step is critical in the performance of a technically sound carotid endarterectomy; if it is done correctly, tacking sutures are rarely required. Meticulous care is then taken to ensure that no loose areas of media remain through the endarterectomized surface. In the author’s practice, Bovine patch angioplasty reapproximates the arteriotomy, and intraoperative duplex ultrasound scanning completes the procedure. The reader is referred to Wylie’s Atlas of Vascular Surgery for color illustrations of the steps used to perform a classic carotid endarterectomy.1
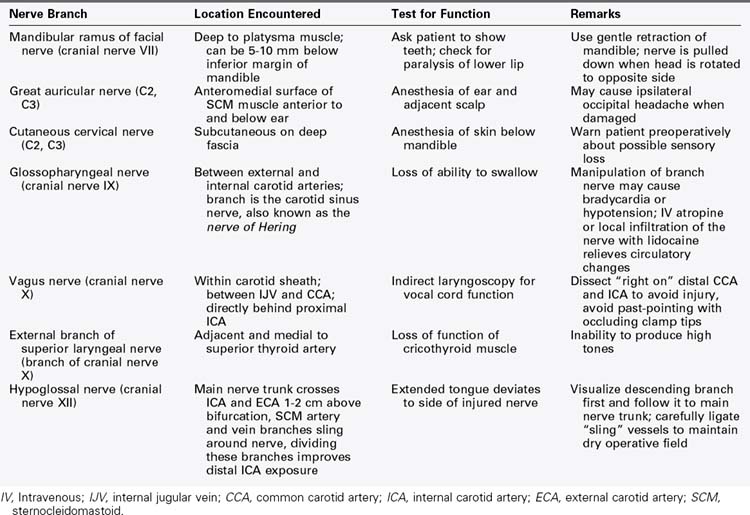
The value of cranial nerve protection during carotid surgery cannot be overemphasized. Despite this admonition, cranial nerve injury (CNI) remains a significant postoperative complication of carotid endarterectomy.2–6 Sajid and colleagues3 reviewed the incidence of CNI after carotid endarterectomy over a 25-year period of time. This metaanalysis included 10,847 patients in 31 studies and compared results that were published before 1995 (15 publications) with those published after 1995 (16 publications). The overall incidence of CNI was 9.4% (1020 injured nerves), and the incidence was higher in publications that occurred before 1995 (10.6% versus 8.3%). Not surprisingly, there was a significant range in the incidence of CNI among different vascular centers, which varied from 1.35% to 31%. The hypoglossal nerve, vagus nerve, and its branches and facial nerves were most often injured, whereas glossopharyngeal and spinal accessory nerve injuries occurred less frequently. Fortunately, almost all (99%) CNIs were transient, and nerve function returned within 3 months with conservative therapy only. Permanent and often disabling CNI occurs with an incidence of 0.5% to 1% after carotid endarterectomy.
In a single-center study published in 1999, Ballotta and colleagues5 reviewed 200 consecutive carotid endarterectomies in Italy. There were 25 cranial nerve injuries (12.5%) in 24 patients, distributed as follows: hypoglossal (11), recurrent laryngeal (8), superior laryngeal (2), marginal mandibular (2), greater auricular (2). Fortunately, the deficits were transient, with all but four resolving by 6 months. The mean recovery time was 5.8 months, with a range of 1 week to 37 months. Forssell and associates6 reviewed 663 consecutive carotid endarterectomy patients in Malmö, Sweden, who were examined preoperatively and postoperatively at the Department of Phoniatrics to determine cranial nerve function. Seventy-five carotid operations (11.4%) resulted in one or more cranial nerve injuries. These injuries included 70 hypoglossal, 8 recurrent laryngeal, 2 glossopharyngeal, and 2 superior laryngeal injuries. Only two nerve injuries (0.30%) were permanent. The frequency of injury increased with a junior surgeon, shunt use, and patch closure.
Exposure of the Distal Internal Carotid Artery
Anatomic dissection in human cadaver specimens demonstrates that division of the posterior belly of the digastric muscle facilitates exposure of the internal carotid artery to the middle of the first cervical vertebra. Anterior subluxation of the mandible improves exposure to the superior border of the first cervical vertebra. The addition of styloidectomy to the maneuvers described previously extends the exposure cephalad, approximately 0.5 cm.7
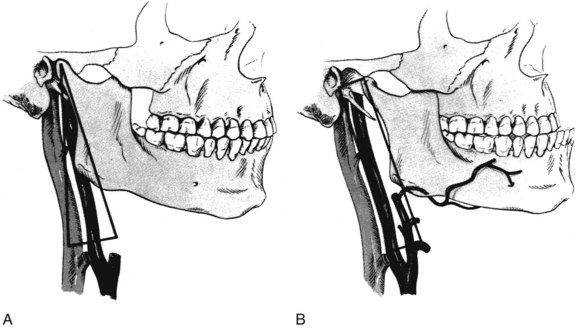
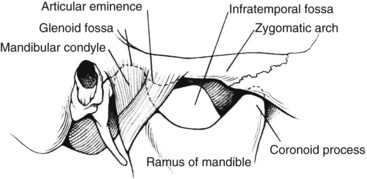
Fisher and associates described a unique technique of wire fixation of the mandible to hold its subluxed position during the operative procedure.8 The 12 to 15 mm of space obtained converts the triangle described earlier into a narrow rectangle (Figure 4-2). It is important to avoid dislocation of the mandible, because serious injury can occur to the temporomandibular joint and even to the contralateral internal carotid artery. In the discussion of Fisher and associates’ paper,8 Stanley suggested that a towel clip placed on the angle of the mandible through two small stab incisions would allow the subluxation to be fixed by minimal retraction. Dossa and associates9 also suggested that temporary mandibular subluxation can be accomplished in a safe and expeditious manner using diagonal, interdental Steinmann pin wiring. Figure 4-3 shows a diagram of the relationship of the mandibular condyle to the auricular eminence and infratemporal fossa.
In situations requiring more room for vascular reconstruction, transection of the mandibular ramus with either translocation or temporary removal of the condyle and ramus fragment affords wider exposure. Wylie and associates10 described this approach and provided detailed color illustrations of the involved anatomy.
Exposure of Aortic Arch Branches and Associated Veins
The most widely accepted direct route for the surgical exposure of the innominate and proximal left common carotid arteries, as well as the superior vena cava and its confluent brachiocephalic veins, is through a full median sternotomy. Although this approach is certainly appropriate in the trauma setting, elective aortic arch branch vessel exposure can be performed with a limited approach. Mini sternotomy is a less invasive surgical exposure for the direct treatment of aortic arch branch vessels and associated major veins.11 Similar to a median sternotomy, this surgical approach provides excellent exposure of the aortic arch branch vessels, with the exception of the left subclavian artery. The first portion of the left subclavian artery is not readily accessible from either anterior approach, because the aortic arch passes obliquely posterior and to the left after its origin from the base of the heart.
Mini sternotomy is performed by first making a limited skin incision measuring 7 to 8 cm in the midline. This incision should extend from the sternal notch to just past the angle of Louis. The manubrium and upper sternum are divided in the midline down to the third intercostal space with a narrow blade mounted on a redo sternotomy oscillating saw (Stryker, Kalamazoo, Mich.). The sternum is then transected transversely at the third intercostal space, creating an upside-down T incision (Figure 4-4). Care is taken not to injure the internal mammary arteries, which are adjacent to the sternum. After accurate hemostasis along the periosteal edges, a Rienhoff or similar pediatric sternal retractor is placed to open the upper sternum. The skin incision can be extended upward along the anterior border of either sternocleidomastoid muscle, with division of the strap muscles to expose the proximal right common carotid artery or the more distal left common carotid artery. This extension can also be used to expose the carotid bifurcation.
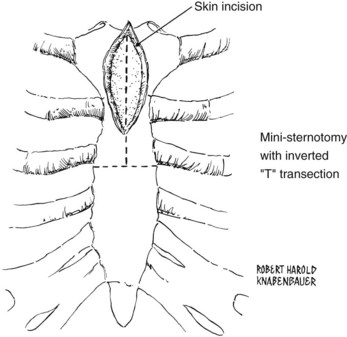
FIGURE 4-4 Skin incision and mini-sternotomy sternal division.
(From Sakopoulos AG, Ballard JL, Gundry SR: Minimally invasive approach for aortic branch vessel reconstruction. J Vasc Surg 31:200, 2000.)
The left brachiocephalic vein can be visualized in the upper portion of the wound. A thymic vein may join this vessel inferiorly, and an inferior thyroid vein may require ligation and division as it joins the brachiocephalic vein superiorly. After complete mobilization of the left brachiocephalic vein, the anterior surface of the aortic arch can be visualized, as well as the origin of the innominate artery. The base of the heart and the innominate and left common carotid arteries are thus exposed (Figure 4-5). The recurrent laryngeal nerve must be protected during exposure of the distal innominate artery. It courses from the vagus nerve anteriorly around the origin of the subclavian artery to return in the tracheoesophageal groove to its termination in the larynx.
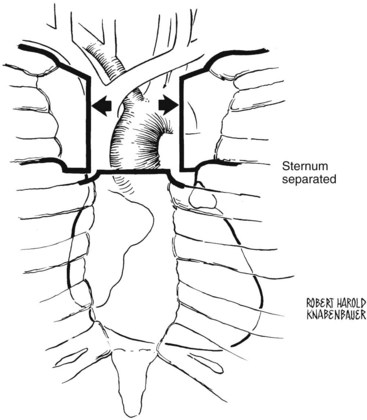
FIGURE 4-5 The upper sternum is divided and separated, exposing the ascending aorta and arch vessels.
(From Sakopoulos AG, Ballard JL, Gundry SR: Minimally invasive approach for aortic branch vessel reconstruction. J Vasc Surg 31:200, 2000.)
Innominate or left common carotid artery endarterectomy, patch angioplasty, or bypass can then be performed in the usual fashion (Figure 4-6). After the procedure, a 19 French Blake drain (Johnson and Johnson, Cincinnati, Ohio) is placed in the mediastinum and brought out laterally through one of the intercostal spaces. This drain is connected to a Heimlich valve grenade suction device. Chest tubes are not used. Two wires are used to bring the upper and lower sternal edges of the T together, and two more are placed in the manubrium. If necessary, another wire placed as a figure eight at the level of the second intercostal space completely rejoins the divided upper sternum. After approximating the muscular and subcutaneous planes in two layers, the skin is closed in a subcuticular fashion.
Exposure of the Origin of the Right Subclavian Artery and Vein
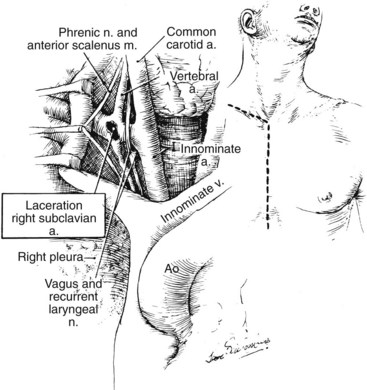
Traumatic vascular injury at the confluence of the subclavian artery and internal jugular and subclavian veins is difficult to manage solely through a supraclavicular approach. Ideally, sternotomy for proximal vascular control should be followed by supraclavicular extension of the incision. However, in the event that the injury is exposed without proximal control, the incision should be promptly extended via a sternotomy while an assistant maintains compression of the vessels against the undersurface of the sternum to temporarily control hemorrhage (Figure 4-7). Alternatively, temporary percutaneous balloon occlusion of the distal innominate artery from a femoral or brachial artery approach can be lifesaving and greatly facilitates this exposure.
Exposure of the Origin of the Left Subclavian Artery
In situations in which there is exigent bleeding into the pleural space from a traumatic injury of the proximal left subclavian artery and percutaneous balloon occlusion is not possible, prompt vascular control can be obtained an anterior thoracotomy in the third or fourth intercostal space. This exposure facilitates placement of a vascular clamp across the origin of the bleeding subclavian artery (Figure 4-8). An inframammary incision is preferred in women, with the breast mobilized superiorly for the exposure just described.
Exposure of the Subclavian and Vertebral Arteries
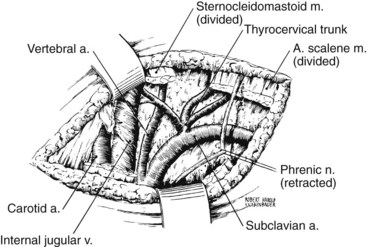
The anterior scalene muscle is divided just above its point of insertion on the first rib to facilitate exposure of the subclavian artery. Division of this muscle should be done under direct vision and without cautery, because the brachial plexus is immediately adjacent to the lateral aspect of the anterior scalene muscle. The origin of the left vertebral artery arises from the medial surface of the subclavian artery medial to the anterior scalene muscle and behind the sternoclavicular joint. The internal thoracic artery, which originates from the inferior surface of the subclavian artery opposite the thyrocervical trunk, should be protected as the subclavian artery is dissected free of surrounding tissue. Figure 4-9 depicts the essential anatomy of this exposure.
The surgical exposure of the distal vertebral artery is described in detail in Chapter 19 of this text and in the surgical literature.12 Injury to the intraosseous portion of the vertebral artery with associated hemorrhage is best managed by embolic occlusion proximal and, if possible, distal to the area of injury.
Exposure of the Axillary Artery
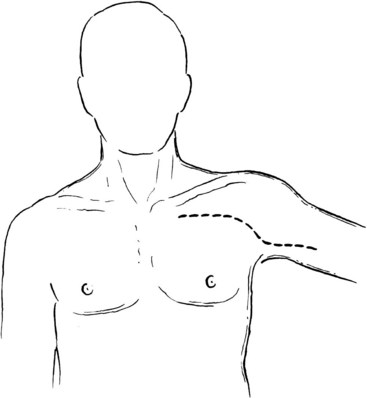
The second portion of the axillary artery is more difficult to expose because it lies directly behind the pectoralis major muscle. Extension of the previously mentioned incision continues across the distal portion of the pectoralis major muscle at the anterior axillary fold and out onto the midline of the proximal medial surface of the arm (Figure 4-10). The tendinous portion of the muscle is divided near its insertion to expose the axillary contents. The pectoralis minor muscle can also be divided if more medial exposure is desired.
Exposure of the Thoracic Outlet
Either a supraclavicular or a transaxillary approach facilitates surgical exposure of the thoracic outlet. Roos described the transaxillary approach for first rib resection in the management of thoracic outlet syndrome.13 However, current treatment approaches for thoracic outlet syndrome favor supraclavicular exposure of the neurovascular structures within the superior thoracic aperture. Essential anatomic elements of this approach have been detailed in Wylie’s Atlas of Vascular Surgery.14
Exposure of the Descending Thoracic and Proximal Abdominal Aorta
No single approach is better for extensive exposure of the thoracic and abdominal aorta than a properly positioned thoracoabdominal incision. After pulmonary artery and radial artery line placement and dual-lumen tracheal intubation, the patient is placed in a modified right lateral decubitus position, with the hips rotated 45 degrees from horizontal. This position allows exposure of both groins if needed. A beanbag device is helpful to support the patient’s position on the operating table. The free left upper extremity should be passed across the upper chest and supported on a cushioned Mayo stand. In this way, thoracoabdominal aortic exposure is gained by unwinding the torso, as described by Stoney and Wylie.15
The extent of thoracic aorta to be exposed will determine which rib interspace to enter. The fourth or fifth intercostal space is used when the entire thoracoabdominal aorta from the subclavian artery origin through the abdominal aorta is to be exposed, whereas the seventh or eighth intercostal space allows mid to terminal thoracic aortic exposure plus wide abdominal aortic visualization. Dividing the respective lower rib posteriorly facilitates this exposure. The thoracic incision is continued across the costal margin in a paramedian plane to the level of the umbilicus (Figure 4-11). If the terminal aorta and iliac vessels are to be exposed, the incision is extended to the left lower quadrant.
The left retroperitoneal space is developed in a retronephric extraperitoneal plane, because surgical exposure of the thoracoabdominal aorta is greatly facilitated by forward mobilization of the left kidney. Division of the median arcuate ligament and lumbar tributary to the left renal vein allows further medial rotation of the abdominal viscera and left kidney. Clearing the posterolateral surface of the thoracoabdominal aorta facilitates aortotomy. With this exposure, the origins of the left renal artery, celiac axis, and the superior mesenteric artery can then be visualized and dissected free of surrounding tissue, as indicated by the disease process present (Figure 4-12). Dissection over the anterior aorta just distal to the left renal artery and underneath the medially rotated left renal vein will bring the right renal artery into view. Alternatively, the origin of this vessel can be readily identified from within the aorta if it is too scarred across the anterior portion of the abdominal aorta or if the aneurysm is too large to safely perform the maneuver described previously.
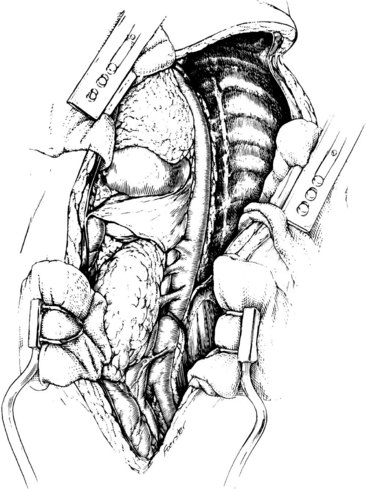
FIGURE 4-12 Thoracoabdominal aortic exposure from the origin of the left subclavian artery to the common iliac arteries.
(From Rutherford RB: Thoracoabdominal aortic exposures. In Rutherford RB, editor: Atlas of vascular surgery: basic techniques and exposures, Philadelphia, 1993, WB Saunders, p 233.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree