Ambulatory and Signal-Averaged Electrocardiography and T-Wave Alternans
Stephen C. Hammill
Overview
Ambulatory electrocardiography (ECG) records a patient’s ECG during routine activities, when the effect of autonomic tone, exercise, emotion, and other stresses can be evaluated. The technique assesses cardiac rhythm, myocardial ischemia, QT-interval duration, signal-averaged ECG, and heart-rate variability. Solid-state recording systems digitize the ECG signal, and all QRS complexes recorded during the 24 hours are available for analysis. These systems have replaced previously used tape systems. Continuous ambulatory recording can now be performed for 1 or more weeks with automatic detection and real-time reporting of life-threatening arrhythmias 24 hours/day. Intermittent and memory-loop ambulatory recorders are helpful for documenting symptoms that occur too infrequently to be assessed by 24-hour ambulatory ECG. The implantable memory-loop recorder is useful in assessing patients with symptoms that occur infrequently and are technically difficult to record with an intermittent or memory-loop recorder.
Ambulatory ECG monitoring in persons without apparent heart disease has identified a wide range of arrhythmias, a finding that must be kept in mind when patients are evaluated. Ambulatory monitoring is helpful when assessing patients with cardiogenic syncope, although memory-loop recorders are of more benefit. Ventricular arrhythmias recorded by 24-hour monitoring after myocardial infarction identify patients at risk for further cardiac events and can be helpful in combination with other prognostic tests in identifying high-risk patients in whom intervention may improve survival. Evaluating therapy using ambulatory monitoring must be done with the knowledge that a significant spontaneous variation occurs in the frequency and complexity of ventricular arrhythmias.
Signal-averaged ECG averages multiple QRS complexes, minimizing the level of noise that contaminates the periodic ECG signal, thereby exposing signals at the microvolt level that are normally hidden within noise. These signals at the end of the QRS complex are termed late potentials and represent delayed conduction through diseased myocardium and a potential physical substrate for reentry ventricular tachycardia. Late potentials usually arise from the border zone surrounding the scar of a previous myocardial infarction, but they can also be present in patients with hypertrophic cardiomyopathy, arrhythmogenic right ventricular dysplasia, myocarditis, and infiltrative heart disease. The use of time-domain analysis to identify late potentials and to predict risk for ventricular arrhythmias has been validated in numerous studies and is the method used in commercial equipment. Frequency-domain analysis is less widely used, and diagnostic endpoints have not been standardized. Signal-averaged ECG using time-domain methods is not reliable in patients with prolonged QRS duration.
The peak incidence of late potentials occurs in the first week after myocardial infarction and slowly decreases during follow-up, with 50% of patients having late potentials before being dismissed from the hospital and 25% having them at 5 years after infarction. Fewer than 5% of patients who do not have late potentials after myocardial infarction develop late potentials during long-term follow-up in the absence of another myocardial infarction. Late potentials are an independent risk factor for the development of ventricular arrhythmias
after myocardial infarction, with a positive predictive accuracy of 20% and a negative predictive accuracy of 95%.
after myocardial infarction, with a positive predictive accuracy of 20% and a negative predictive accuracy of 95%.
T-wave alternans (TWA) is an ECG pattern characterized by changing morphology of the T wave on an every-other-beat basis. TWA is rarely seen on the ECG, but, when seen, is associated with pathophysiologic conditions that may give rise to ventricular arrhythmias, including Prinzmetal angina, electrolyte abnormalities, acute ischemia, and long-QT syndrome. With the advent of advanced signal-processing methods, TWA can now be measured at the microvolt level, even though visual inspection of the ECG shows no abnormality. Studies have demonstrated that microvolt-level TWA is a predictor of susceptibility to ventricular arrhythmias. TWA is caused by local alteration in the duration of the action potential as documented during electrophysiologic mapping studies. The development of reentrant ventricular arrhythmias, including ventricular tachycardia and ventricular fibrillation, is hypothesized to be caused by local action potential alternans, which leads to spatial dispersion of recovery and the fractionation of depolarizing wavefronts, allowing reentry. Recent literature has supported the use of microvolt-level TWA to identify patients at risk for life-threatening ventricular arrhythmias and sudden cardiac death and, possibly, which patients will benefit from primary prevention of sudden death using the implantable defibrillator.
Glossary
Complex ventricular ectopy
Nonsustained ventricular tachycardia, more than 10 premature ventricular complexes per hour.
Continuous ambulatory ECG
ECG recording for 24 to 48 hours that stores every heartbeat.
Delayed ventricular potentials
Another term for late potentials.
FQRSD
Filtered QRS duration. The total duration of the QRS complex is measured after filtering to determine whether late potentials are prolonging the QRS complex.
Frequency-domain signal-averaged ECG
A method of assessing late potentials by mathematical methods to measure frequency content of the QRS waveform.
High-resolution ECG
Another term for signal-averaged ECG.
Intermittent ambulatory ECG
ECG recording in which ECG data are stored only when the device is activated. Data are stored after the device is activated.
k
The alternans ratio used to define whether TWA is present.
LASD
Low-amplitude signal duration. The terminal QRS below 40 μV is measured to assess whether low-level late potentials are present.
Late potentials
Low-level electrical signals at the end of the QRS complex that represent slow conduction through abnormal myocardium.
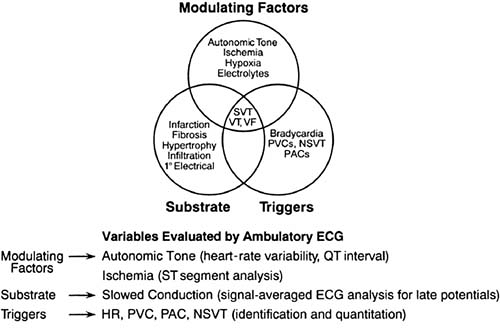
Modulating factor
Variable that interacts with triggers and substrate to initiate an arrhythmia. Modulating factors include electrolytes, ischemia, and autonomic tone.
Memory-loop ambulatory ECG
ECG recordings taken for several days to weeks with ECG data stored to permanent memory only when the device is activated. The ECGs preceding and following device activation are stored.
RMSV
Root mean square voltage. The last 40 msec of the QRS complex is assessed to determine whether a late potential is present.
Signal-averaged ECG
The standard ECG is filtered, and multiple QRS complexes are averaged, allowing identification of late potentials.
Time-domain signal-averaged ECG
A method of assessing late potentials by measuring their duration and amplitude.
Trigger
Cardiac events that initiate a sustained arrhythmia. Triggers include premature atrial and ventricular extrasystoles and alteration in cycle length.
TWA
T-wave alternans.
Valt
The alternans voltage used to define whether TWA is present.
Ambulatory Electrocardiography
Historical Perspective
Ambulatory ECG was introduced by Holter and Gengerelli in 1949 (1). During the subsequent four decades, extensive clinical experience has developed, and ambulatory ECG monitoring has demonstrated that the test is useful for the diagnosis and management of cardiac arrhythmias and for assessing prognosis in populations of patients at high risk for life-threatening cardiac arrhythmias (2).
The ambulatory ECG was initially used to assess patients for the presence of tachycardia or bradycardia. The technique evolved to be used for evaluation of myocardial ischemia by recording changes in the ST segment, or the QT interval duration as a prognostic factor for serious ventricular arrhythmia, of signal-averaged ECG to identify late potentials and substrate for serious ventricular arrhythmias, and of changes in the RR interval, reflecting heart-rate variability, a strong prognostic indicator for serious cardiac events.
The equipment used to record the ambulatory ECG has also evolved, with improved recording fidelity, reduction in size and weight, improved data acquisition using solid-state technology, and use of computer-enhanced analysis systems. In addition, patient-activated event recorders allow intermittent ECG recording at the time that infrequent symptoms occur. The implantable automatic and patient-activated ECG recording system allows documentation of the cardiac rhythm during symptomatic episodes that may occur as infrequently as every 12 months.
Pathophysiology of Cardiac Arrhythmias
The ambulatory ECG records the effect of changing autonomic tone and environmental factors on the development of cardiac arrhythmias. Sustained tachycardia depends on the interaction of triggers, substrate, and modulating factors (3) (Figs. 60.1 and 60.2).
Continuous Ambulatory Electrocardiographic Monitoring
The two common recording systems are tape-based and solid-state systems. The tape-based system records at an extremely slow tape speed, with two or three ECG amplifiers and an analogue tape recorder. Irregularities in the tape drive can create artifacts that simulate tachycardia or bradycardia. Tape-based systems have been replaced by solid-state recording systems. Solid-state recording systems digitize the incoming ECG signal from two or three channels and then store this data in the solid-state memory. The digital signal is assessed by intricate algorithms to determine whether the QRS complex is normal or represents an ectopic beat or part of a run of tachycardia. Such systems combine the advantages of the tape-based system, in which all ECG complexes are recorded and analyzed, with the advantages of a smaller, quieter, and easier-to-maintain solid-state system. Solid-state devices depend on efficient and accurate algorithms (4,5).
Intermittent and Memory-Loop Ambulatory Electrocardiographic Recording
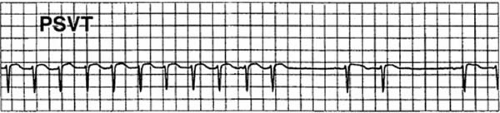
Intermittent recorders are capable of storing only a limited number of short, patient-activated ECG recordings (12) (Fig. 60.3). These devices are lightweight, and some are as small as a thick credit card. Their advantage over continuous ambulatory systems is that the ECG is more likely to be obtained while the patient is experiencing clinical symptoms, thus allowing a direct correlation between the patient’s symptoms and the ECG recorded at that instant (6,7,8). Current devices use solid-state technology that allows recording of 30 to 60 seconds of ECG for three to six occasions when the patient is feeling symptoms. The ECG is stored in the solid-state memory of the device and
transmitted by telephone to the base station at a time that is convenient for the patient.
transmitted by telephone to the base station at a time that is convenient for the patient.
Development of this technology led to memory-loop circuitry, which is a hybrid between the ambulatory ECG monitor and the solid-state event recorder. With the memory-loop recorder, electrodes are applied to the patient and the device is worn on a continuous basis. When the patient activates the device, a permanent recording of stored ECG data is obtained that includes periods from 1 to 4 minutes before and 30 to 60 seconds after the recording button is pressed. The data can then be transmitted by telephone at the patient’s convenience. This device has the disadvantage that it must be worn on a continuous basis but the clear advantage that it obtains ECG data recorded before the patient presses the button and thus documents the rhythm at the onset of the patient’s palpitations and the initiating event (9). Approximately 25% of patients who have recurrent syncope obtain diagnostically useful information using this device. Memory-loop recorders are limited by malfunction and by the inability of 20% of patients to properly use the device (10).
The implantable memory-loop recorder is placed subcutaneously in the left upper chest, allowing continuous ECG recording using a loop-based memory system capable of recording for up to 15 months (11). The device can be activated by the patient or set to automatically record an arrhythmia that is below or above a predetermined heart rate. The data are then downloaded into a computer for analysis and review. This system has the unique advantage of not being dependent on patient interaction, avoiding problems caused by patients who are unable to activate the device or place the recording leads. The device can be used for extended periods of time, as opposed to the standard memory-loop recorder, which is less practical after 3 to 4 weeks because of skin irritation from the patch electrodes. The implantable memory-loop recorder also allows the ECG to be recorded while the patient is exposed to water, when other systems cannot be used. A report of 85 patients who had syncope and negative ambulatory monitoring, tilt-table and electrophysiologic testing, showed that 58 patients (68%) had recurrent syncope at a mean of 10.5 months after implantation (12). The diagnosis of the syncopal mechanism included bradycardia in 18 patients and tachycardia in 3 patients; normal rhythm was recorded at the time of syncope in the remaining patients, suggesting a vasodepressor mechanism.
The most recent development in this technology involves continuous ECG recording in an ambulatory setting with real-time analysis of the recorded data 24 hours/day, 7 days/week. Serious arrhythmias detected by the device are transmitted to a base station and then to the analysis center. The patient’s physician is notified that a serious rhythm disturbance has occurred. This device is useful for home monitoring while medications are being started to control an arrhythmia, adjusted to control heart rate during atrial fibrillation, or detection of arrhythmia after myocardial infarction (13).
Selection of 24-hour continuous ambulatory versus intermittent ECG recording depends on the patient and the symptoms (14). Both recording systems are most useful when a patient has typical symptoms while the ECG is being recorded. The absence of any significant rhythm abnormality while symptoms are occurring is helpful in patient management because it eliminates arrhythmia as a possible cause of the symptoms.
Patients with infrequent symptoms are more likely to benefit from the intermittent or memory-loop recorder. The memory-loop recorder is most useful for patients who are expected to have symptoms within 4 weeks after they begin using the device and in whom symptoms are brief and not expected to last long enough to find, apply, and record with the intermittent recorder. The implantable memory-loop recorder is helpful for patients who have syncope or near-syncope of unknown etiology despite standard evaluation but who have spells too infrequently to justify a standard memory-loop recorder (15,16).
Ambulatory Electrocardiographic Findings in Normal, Asymptomatic Persons
Ambulatory ECG monitoring in persons without apparent heart disease has identified a wide range of arrhythmias (Table 60.1) (17,18,19,20,21). The frequency of arrhythmias increases with age (22,23,24,25). In athletes (26,27), 24-hour ambulatory ECG monitoring, in comparison to controls, identified a significantly lower average minimum heart rate (38 vs. 45 beats per minute), more sinus pauses that were longer than 2 seconds (37% vs. 6%), more episodes of Mobitz I block (23% vs. 6%), and more junctional rhythm (20% vs. 0%), but no significant difference in the number of premature ventricular complexes (PVCs) (33% vs. 43%). The results of ambulatory ECG monitoring should be placed in context with the patient’s clinical condition and associated symptoms.
Ambulatory Recordings in Specific Clinical Syndromes
Cardiogenic Syncope
Patients who experience sudden loss of consciousness are best evaluated with techniques other than ambulatory monitoring because of the severity of their symptoms. Such patients may be admitted to the hospital for long-term ECG assessment and consideration for electrophysiologic testing, tilt-table testing, or exercise assessment to identify the cause of their spells. Patients with syncope who undergo ambulatory ECG monitoring experience lightheadedness, presyncope, or syncope during the recording 25% of the time (28,29). Approximately 2% to 15% are identified as having an associated cardiac arrhythmia when the symptoms occurred, and 35% have no associated ECG abnormality (21,29,30). Extending the recording to 3 days
increases the number of patients who experience symptoms to 50% (31).
increases the number of patients who experience symptoms to 50% (31).
TABLE 60.1 Arrhythmia Occurrence During Ambulatory Electrocardiogram Monitoring in Healthy Patients | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The criteria for determining a significant arrhythmia in the absence of associated symptoms are difficult to establish. For example, many authors indicate that a sinus pause of 2.0 to 2.5 seconds is abnormal, but other authors have shown that sinus pauses of 3 seconds or longer may not cause symptoms and do not predict an adverse prognosis (32,33). Therefore, a sinus pause should be correlated with symptoms before intervention is considered.
Patients who have cardiogenic syncope and associated heart disease, cardiac arrhythmia on ambulatory ECG monitoring, or abnormal 12-lead ECG results should be considered for electrophysiologic testing. Patients without these abnormalities are generally considered for tilt-table testing. Thirty percent of patients with negative electrophysiologic and tilt-table studies continue to have syncope and may benefit from use of a memory-loop recorder. The implantable memory-loop recorder is uniquely beneficial in this group of patients.
Atrial Fibrillation
In patients with paroxysmal atrial fibrillation, 24-hour ambulatory ECG monitoring can help to identify the onset of the arrhythmia and potential approaches to treatment including vagal-dependent atrial fibrillation (34) and atrial fibrillation caused by an episode of atrial flutter or supraventricular reentrant arrhythmia. The 24-hour ambulatory recording system is also helpful in assessing a patient’s rate control during atrial fibrillation. Patients may demonstrate adequate rate control while they are sedentary but experience a rapid heart rate and symptoms when they are active. Finally, 24-hour ambulatory monitoring may be helpful in identifying patients in whom a pulmonary vein focus is the cause of atrial fibrillation and would benefit from catheter ablation to isolate the pulmonary veins.
Ventricular Arrhythmias after Myocardial Infarction
The role of complex ventricular ectopy in predicting mortality after myocardial infarction has been confirmed in several multicenter trials (35,36,37,38,39) (Table 60.2). A 1996 study by Statters and associates (38) reported that PVCs are an independent predictor of mortality after myocardial infarction in patients who undergo thrombolytic therapy. The positive predictive accuracy of PVCs in predicting cardiac mortality and arrhythmic events in patients receiving thrombolytic therapy was 19.4% and 25.8%, respectively, compared to 16% and 16%, respectively, for patients who did not receive thrombolytic therapy.
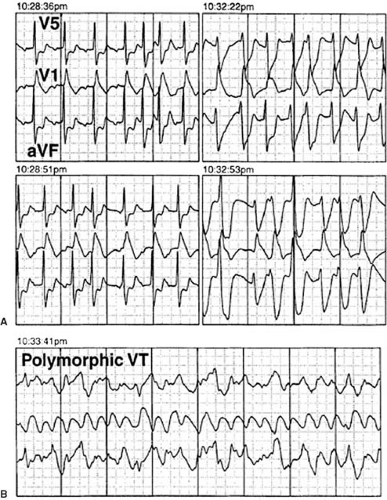
Studies of this nature emphasize the importance of PVC triggers associated with structural heart disease in the development of life-threatening ventricular arrhythmias after myocardial infarction (40). Risk factors that can be assessed by 24-hour ambulatory monitoring, including PVC frequency, abnormal autonomic tone assessed by reduced heart-rate variability, and ischemia monitoring of ST-segment changes, further delineate the dynamic relationship among PVC trigger, substrate, and modulating factors that can converge to create a life-threatening arrhythmic event (Fig. 60.4). Nonsustained ventricular tachycardia (NSVT) with reduced ejection fraction has been used to identify postinfarction patients who are at potential risk for a cardiac event and would benefit from further evaluation by means of electrophysiologic testing or placement of the implantable cardioverter-defibrillator (2).
Dilated and Hypertrophic Cardiomyopathy
Ventricular arrhythmias recorded by 24-hour ambulatory ECG monitoring independently predict subsequent death in patients with dilated cardiomyopathy (41,42). The use of angiotensin-converting-enzyme inhibitors has significantly increased survival in patients with moderately symptomatic congestive heart failure and reduced ventricular function. Nonetheless, approximately 25% to 50% of deaths in patients with congestive heart failure are related to cardiac arrhythmias, despite the use of angiotensin-converting-enzyme inhibitors (43,44).
In 1981, NSVT recorded during 24-hour ambulatory monitoring was reported to be associated with sudden death (45,46), with an 8% annual mortality rate in patients with hypertrophic cardiomyopathy. A subsequent study by Spirito and associates (47) reviewed 151 asymptomatic patients with hypertrophic cardiomyopathy in whom the finding of NSVT on ambulatory monitoring was associated with a relative risk of sudden death of 2.4 compared with patients without NSVT. Although the risk of sudden death was slightly higher in the presence of NSVT, these authors did not recommend that a finding of NSVT should lead to the initiation of antiarrhythmic therapy but should alert the physician that further evaluation of the patient is needed.
Evaluating Therapy
Twenty-four-hour ambulatory ECG monitoring is useful in assessing the response to antiarrhythmic therapy and the performance of a permanent pacemaker (48,49,50,51). Ambulatory monitoring is useful in patients with atrial fibrillation, atrial flutter, and atrial tachycardia to determine whether adequate rate control is achieved with atrioventricular (AV) nodal–blocking drugs during activity and periods of emotional stress while avoiding prolonged pauses during periods of inactivity or sleep. Questions of pacemaker function are often assessed with 24-hour ambulatory monitoring. Failure to sense or pace that is not easily detected during a brief interrogation of the pacemaker may be apparent during 24-hour monitoring.
Heart-Rate Variability, ST-Segment Changes, and QT Interval
Ambulatory ECG monitoring has been found to be helpful for assessing autonomic tone, through measurement of RR intervals and assessment of heart-rate variability (see Chapter 77) and for ascertaining the frequency of silent and symptomatic myocardial ischemia through ST-segment analysis (see Chapter 18). QT-interval prolongation is a marker of repolarization changes associated with malignant ventricular arrhythmia, termed torsades de pointes (52,53,54).
Personal Perspectives
Use of multiday, real-time ambulatory ECG to assess rhythm abnormalities as a cause of intermittent symptoms and to adjust medications will increase, as will the use of memory-loop recorders that are either worn externally or implanted.
Conclusions
Ambulatory ECG monitoring is an established clinical test with demonstrated usefulness for evaluating patients who have arrhythmia and ischemia and for assessing factors associated with increased risk of sudden death. Technological advances will continue to enhance ambulatory ECG, making reductions in the size of recorders, longer recording periods, and more rapid and accurate analysis possible. Memory-loop recorders will play an increasingly important role in assessment of patients who have intermittent symptoms that suggest cardiac arrhythmia. The implantable memory-loop recorder will be used commonly in patients who have infrequent episodes of near-syncope and syncope. Continuous real-time ambulatory
monitoring with arrhythmia detection and physician notification 24 hours/day, 7 days/week will increase in use.
monitoring with arrhythmia detection and physician notification 24 hours/day, 7 days/week will increase in use.
TABLE 60.2 Frequency of Premature Ventricular Complexes and Relationship to Mortality in Patients With Myocardial Infarction | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Signal-Averaged Electrocardiography
Historical Perspective
Signal averaging of the surface ECG is a noninvasive computerized method of analyzing standard ECGs that identifies patients who are at risk for ventricular tachycardia (55,56,57). This high-resolution ECG technique averages multiple QRS complexes, minimizing the level of noise that contaminates the periodic ECG signal, thereby exposing signals at the microvolt level that are normally hidden within noise (56). The technique identifies electrical signals at the end of the QRS complex, termed late potentials, which represent delayed conduction through diseased myocardium and thus a potential physical substrate for reentrant ventricular tachycardia. Late potentials have been correlated with clinical ventricular tachycardia, ventricular tachycardia inducibility at the time of electrophysiologic testing, and arrhythmic events after myocardial infarction (57,58).
Signal averaging was first used in ECG to noninvasively record a bundle of His electrogram (59,60). Han (61) then demonstrated in 1969 that electrical activity may persist beyond the QRS complex after myocardial infarction in the canine heart. These potentials were referred to as delayed ventricular potentials and were recorded by Durrer et al. in 1971 (62). The relationship of late potentials to the genesis of ventricular arrhythmias was subsequently demonstrated in the early 1970s (63,64,65,66). These technical advances introduced time-domain analysis of the signal-averaged ECG and subsequent correlation of late potentials with ventricular arrhythmias.
Pathophysiology of Late Potentials
In 1973, Boineau and Cox (66) demonstrated that electrograms recorded from ischemic regions of the canine myocardium were fractionated and delayed beyond the QRS complex and into the ST segment. Delayed activation is the substrate for reentrant ventricular tachycardia in patients with acute or remote myocardial infarction (63,64,65). Josephson and associates (68) described intracardiac recordings of low-level diastolic electrical activity during ventricular tachycardia, representing reentry within the border zone of the infarction. The low-level late potentials recorded directly from the heart can also be recorded from the body surface using a method of high amplification, band-pass filtering, and signal averaging (69).
Late potentials are usually found in border zones surrounding the scar of a previous myocardial infarction. Fibrosis forms an insulating boundary between muscle bundles and Purkinje tissue that slows and fragments the wave of electrical depolarization as it sweeps through the ventricular myocardium, constituting the substrate for reentry. Endocardial recordings of electrical depolarization from a border zone demonstrate conduction delay and fragmentation (Fig. 60.5). The border zone is both the source of late potentials and the substrate
for reentrant ventricular tachycardia. Signal-averaged ECG has subsequently been demonstrated to record delayed electrical activation through the myocardium in several pathologic conditions in addition to myocardial ischemia including hypertrophic cardiomyopathy, dilated cardiomyopathy, arrhythmogenic right ventricular dysplasia, myocarditis, and infiltrative heart disease. The technique has been extended to the atrium, where measures of atrial conduction delay correlate with the development of atrial fibrillation.
for reentrant ventricular tachycardia. Signal-averaged ECG has subsequently been demonstrated to record delayed electrical activation through the myocardium in several pathologic conditions in addition to myocardial ischemia including hypertrophic cardiomyopathy, dilated cardiomyopathy, arrhythmogenic right ventricular dysplasia, myocarditis, and infiltrative heart disease. The technique has been extended to the atrium, where measures of atrial conduction delay correlate with the development of atrial fibrillation.
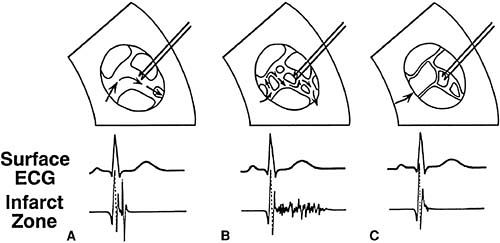 FIGURE 60.5. Late potentials occurring in the border zone of experimental myocardial infarction in the canine model. A: No conduction delay with a normal QRS complex recorded by both the surface electrocardiogram (ECG) and the electrode from the infarct zone. B:
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|