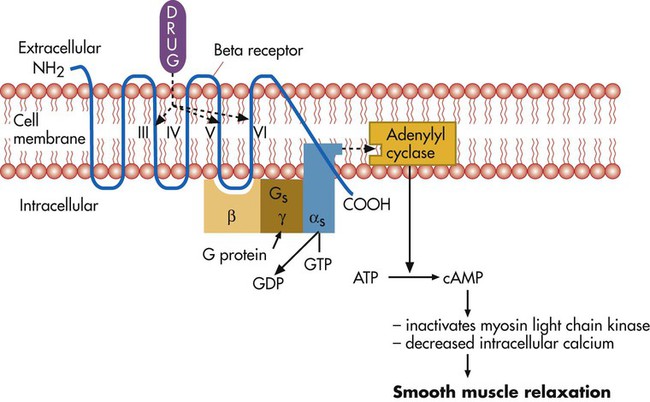
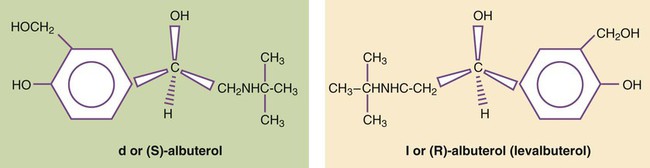
After reading this chapter you will be able to: The primary focus of respiratory care pharmacology is the delivery of bronchoactive inhaled aerosols to the respiratory tract for the diagnosis and treatment of pulmonary diseases. Although other drug classes are used in respiratory care, discussion in this chapter is limited to bronchoactive inhaled aerosols. Other drug classes are reviewed in pharmacology texts.1,2 The drug administration phase describes the method by which a drug dose is made available to the body. Administering drugs directly to the respiratory tract uses the inhalation route, and the dose form is an aerosol of liquid solutions, suspensions, or dry powders. The most commonly used devices to administer orally or nasally inhaled aerosols are the metered dose inhaler (MDI), the small volume nebulizer (SVN), and the dry powder inhaler (DPI). Reservoir devices, including holding chambers with one-way inspiratory valves and simple, nonvalved spacer devices, are often added to MDIs to reduce the need for complex hand-breathing coordination and to reduce oropharyngeal impaction of the aerosol drug (see Chapter 36). The advantages of treatment of the respiratory tract with inhaled aerosols are as follows: • Aerosol doses are usually smaller than doses for systemic administration. • Onset of drug action is rapid. • Delivery is targeted to the organ requiring treatment. Disadvantages of the delivery of inhaled aerosols in treating respiratory disease include the number of variables affecting the delivered dose and lack of adequate knowledge of device performance and use among patients and caregivers.3 The mechanisms of drug action are briefly described for each class of bronchoactive drug. • Adrenergic (adrenomimetic): Drug that stimulates a receptor responding to norepinephrine or epinephrine • Antiadrenergic: Drug that blocks a receptor for norepinephrine or epinephrine • Cholinergic (cholinomimetic): Drug that stimulates a receptor for acetylcholine • Anticholinergic: Drug that blocks a receptor for acetylcholine • Muscarinic: Drug that stimulates acetylcholine receptors specifically at parasympathetic nerve–ending sites Because cholinergic receptors exist at autonomic ganglia and at the myoneural junction in skeletal muscle, the terms muscarinic and antimuscarinic distinguish cholinergic agents whose action is limited to parasympathetic sites. Neostigmine is a cholinergic (indirect-acting) drug that increases receptor stimulation at both the myoneural junction and the parasympathetic sites. By contrast, atropine is an antimuscarinic agent, which blocks the action of acetylcholine only at the parasympathetic sites. Table 32-1 summarizes receptors and their effects for the cardiopulmonary system. A more detailed description of the autonomic nervous system and receptor subtypes is provided by Katzung and colleagues.2 TABLE 32-1 Airway Receptors and Their Effects in the Cardiopulmonary System* M2, M3, Subtypes of muscarinic (M) cholinergic receptors. *Adrenergic and muscarinic cholinergic receptor subtypes are indicated. Adrenergic bronchodilators represent the largest group of drugs among the aerosolized agents used for oral inhalation. Table 32-2 lists bronchodilators in this group, with their aerosol formulations, selected brand names, and dosages. TABLE 32-2 Adrenergic Bronchodilator Agents Available in the United States Short-acting beta-2 agonists, such as albuterol and levalbuterol, are indicated for relief of acute reversible airflow obstruction in asthma or other obstructive airway diseases. Short-acting agents are termed rescue agents in the 2007 National Asthma Education and Prevention Program Expert Panel III (NAEPP EPR III) guidelines.6 • Alpha-receptor stimulation: Causes vasoconstriction and a vasopressor effect (increased blood pressure) • Beta-1-receptor stimulation: Causes increased heart rate and myocardial contractility • Beta-2-receptor stimulation: Relaxes bronchial smooth muscle, stimulates mucociliary activity, and has some inhibitory action on inflammatory mediator release Bronchodilation, through stimulation of beta-2 receptors, is the desired therapeutic effect. Both alpha-adrenergic and beta-adrenergic receptors are G protein–linked receptors. Figure 32-2 illustrates the mode of action for relaxation of airway smooth muscle when a beta-2 receptor is stimulated. The nature of the beta receptor and its activity is presented in detail in a review by Barnes.7 Adrenergic bronchodilator agents represent the evolution of a drug class. Although all of these agents are adrenergic agonists, the differences among individual agents are due to their receptor preference (alpha-adrenergic, beta-1-adrenergic, beta-2-adrenergic) and their different pharmacokinetics as listed in Table 32-2. These differences determine the optimal clinical application of individual agents, as discussed subsequently. The adrenergic bronchodilators form three subgroups. Levalbuterol is approved as a single-isomer beta-2-selective agonist. Previous inhaled formulations of adrenergic bronchodilators all were synthetic racemic mixtures, containing both the (R)-isomer and the (S)-isomer in equal amounts. Levalbuterol is the pure (R)-isomer of racemic albuterol. Both stereoisomers of albuterol are shown in Figure 32-3 with the single-isomer (R-isomer) form of levalbuterol. Although the (S)-isomer is physiologically inactive on adrenergic receptors, there is accumulating evidence that the (S)-isomer is not completely inactive. Box 32-1 lists some of the physiologic effects of (S)-albuterol noted in the literature.8–14 The effects noted antagonize the bronchodilating effects of the (R)-isomer and promote bronchoconstriction. In addition, the (S)-isomer is more slowly metabolized than the (R)-isomer. Levalbuterol is available as a nebulization solution in three strengths: 0.31 mg/3 ml, 0.63 mg/3 ml, and 1.25 mg/3 ml. As a result of mixing of other inhaled agents, levalbuterol is also available as a concentrate of 1.25 mg/0.5 ml and an MDI. In a study by Nelson and associates,15 the 0.63-mg dose was found to be comparable to the 2.5-mg racemic albuterol dose in onset and duration. Side effects of tremor and heart rate changes were less with the single-isomer formulation. The 1.25-mg dose showed a higher peak effect on forced expiratory volume in 1 second (FEV1) with an 8-hour duration compared with racemic albuterol. Side effects with this dose were equivalent to the side effects seen with racemic albuterol. An equivalent clinical response was seen with one-fourth of the racemic dose (0.63 mg) using the pure isomer, although the racemic mixture contains 1.25 mg of the (R)-isomer (half of the total 2.5-mg dose). A detailed review is available of levalbuterol and differences between the (R)-isomer and (S)-isomer of albuterol.16 The release of salmeterol offered the first long-acting adrenergic bronchodilator in the United States. In contrast to previous agents, the duration of action of salmeterol is about 12 hours. The pharmacokinetics of salmeterol makes it suitable for maintenance therapy, in particular, with nocturnal asthma. However, it is not well suited for relief of acute airflow obstruction or bronchospasm because its onset is longer than 20 minutes, with a peak effect occurring by 3 to 5 hours. Although this agent is a beta-2 agonist, its exact mode of action differs from previous beta-2 agonists, allowing persistent receptor stimulation over a prolonged period of hours. A more detailed discussion of the action of salmeterol can be found in a review by Johnson and colleagues.17 Formoterol is a second long-acting, beta-2-specific agent and is approved for general clinical use in the United States. The duration of effect is approximately 12 hours, but in contrast to salmeterol, the onset of action and peak effect of formoterol are rapid and similar to albuterol.18 Nonetheless, patients should be cautioned about the risk of accumulation and toxicity if formoterol is used as a rescue agent in the same way that shorter acting beta agonists, such as albuterol, are used. As with salmeterol, the extensive side chain or tail makes formoterol more lipophilic than shorter acting bronchodilators and is the basis for its longer duration of effect. A new novel once-daily, long-acting beta-agonist, indacaterol, was approved in the United States in 2011 under the brand name of Arcapta Neohaler. It has been used mainly to treat asthma, and additional indications for COPD are being examined. Indacaterol is also being studied in combination with tiotropium bromide with successful preliminary outcomes.1 The implication of beta-2-adrenergic agonists in deaths from asthma—termed the asthma paradox or the beta agonist controversy—remains debated.19 There is evidence of loss of a bronchoprotective effect with use of beta agonists, and patients should be cautioned to avoid asthma triggers.20 The increased prevalence of asthma in general remains a troublesome and unresolved issue. • Monitor flow rates using bedside peak flowmeters, portable spirometry, or laboratory reports of pulmonary function before and after bronchodilator studies to assess reversibility of airflow obstruction. • Assess arterial blood gases or pulse oximetry saturation, as needed, for acute states with asthma or COPD to monitor changes in ventilation and gas exchange (oxygenation). • Note the effect of beta agonists on blood glucose (increase) and K+ (decrease) laboratory values, if using high doses, such as with continuous nebulization or emergency department treatments. • In the long-term, monitor pulmonary function studies of lung volumes, capacities, and flows. • Instruct asthmatic patients in the use and interpretation of disposable peak flowmeters to assess severity of asthmatic episodes and provide an action plan for treatment modification. • Emphasize in patient education that beta agonists do not treat underlying inflammation and do not prevent progression of asthma, and additional antiinflammatory treatment or more aggressive medical therapy may be needed if there is a poor response to the rescue beta agonist. • Instruct and then verify correct use of aerosol delivery device (SVN, MDI, reservoir, DPI). • Instruct patients in use, assembly, and especially cleaning of aerosol inhalation devices. The following actions are suggested to evaluate patient response to long-acting beta agonists: • Assess ongoing lung function, including predose FEV1 over time and variability in peak expiratory flows. • Assess amount of rescue beta agonist use and nocturnal symptoms. • Assess number of exacerbations, unscheduled clinic visits, and hospitalizations. • Assess days of absence from school or work because of symptoms. • Assess ability to reduce the dose of concomitant inhaled corticosteroids. • Long-acting beta-2 agonists are not to be used without a controller medication (i.e., corticosteroid). • Long-acting beta-2 agonists should not be used by patients who are controlled on low-dose or medium-dose inhaled corticosteroids. • Long-acting beta-2 agonists should be used only if patients are not controlled with agents such as inhaled corticosteroids. • Long-acting beta-2 agonists should be for short-term use only. A long-acting beta-2 agonist should be discontinued when asthma is controlled. • Children should use a long-acting beta-2 agonist only in conjunction with a corticosteroid. The use of a combination product is needed to increase adherence. Ipratropium bromide and tiotropium bromide are the only inhaled anticholinergic bronchodilators available in the United States. Table 32-3 lists the dosage forms and pharmacokinetics of ipratropium and tiotropium. Generally, anticholinergic agents have been found to be as effective as beta agonists in airflow improvement in COPD but less so in asthma. A nasal formulation of ipratropium is also available for relief of allergic and nonallergic perennial rhinitis, including the common cold.21 TABLE 32-3 Inhaled Anticholinergic Bronchodilator Agents*
Airway Pharmacology
 Analyze three phases that constitute the course of drug action from dose to effect.
Analyze three phases that constitute the course of drug action from dose to effect.
 Describe classes of drugs that are delivered via the aerosol route.
Describe classes of drugs that are delivered via the aerosol route.
 Compare mode of action, indications, and adverse effects that characterize each major class of aerosolized drug.
Compare mode of action, indications, and adverse effects that characterize each major class of aerosolized drug.
 Compare available aerosol formulations, brand names, and dosages for each specific drug class.
Compare available aerosol formulations, brand names, and dosages for each specific drug class.
 Select the appropriate drug class for a specific patient or clinical situation.
Select the appropriate drug class for a specific patient or clinical situation.
Principles Of Pharmacology
Drug Administration Phase
Pharmacodynamic Phase
Signaling Mechanism
Example
Mediation by G protein (guanine nucleotide)–linked receptors
Beta-adrenergic agonists, antimuscarinic agents
Attachment to intracellular receptors by lipid-soluble drugs
Corticosteroids
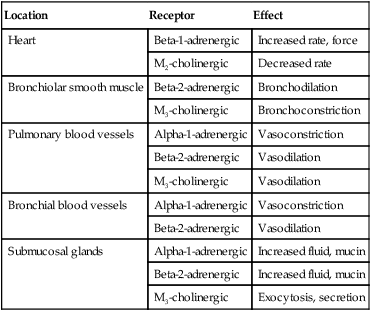
Airway Receptors and Neural Control of the Lung
Location
Receptor
Effect
Heart
Beta-1-adrenergic
Increased rate, force
M2-cholinergic
Decreased rate
Bronchiolar smooth muscle
Beta-2-adrenergic
Bronchodilation
M3-cholinergic
Bronchoconstriction
Pulmonary blood vessels
Alpha-1-adrenergic
Vasoconstriction
Beta-2-adrenergic
Vasodilation
M3-cholinergic
Vasodilation
Bronchial blood vessels
Alpha-1-adrenergic
Vasoconstriction
Beta-2-adrenergic
Vasodilation
Submucosal glands
Alpha-1-adrenergic
Increased fluid, mucin
Beta-2-adrenergic
Increased fluid, mucin
M3-cholinergic
Exocytosis, secretion
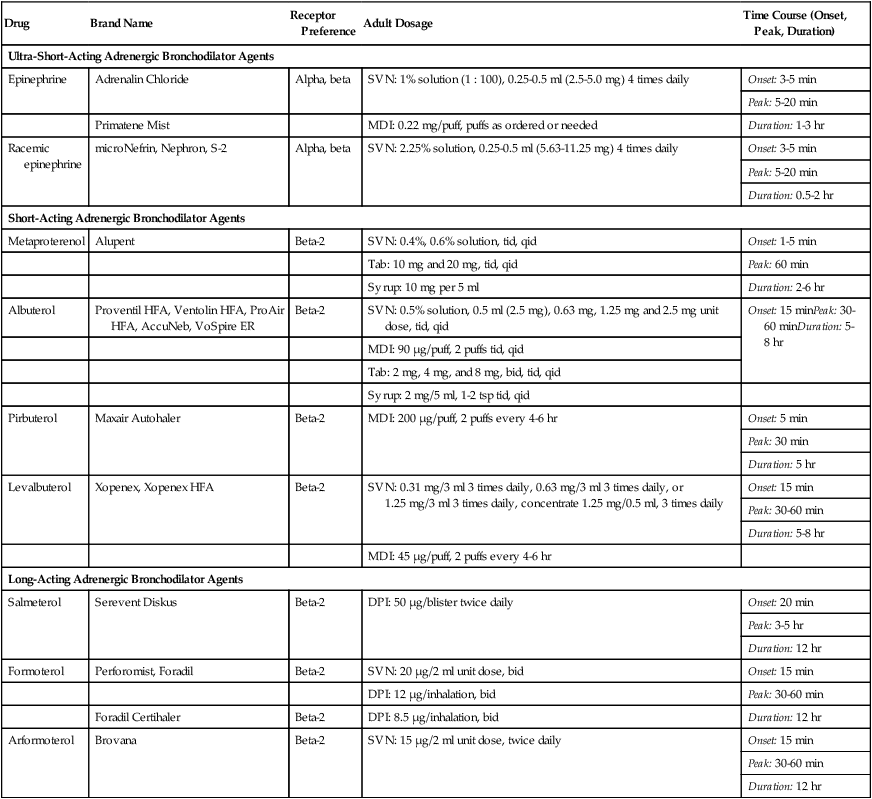
Adrenergic Bronchodilators
Drug
Brand Name
Receptor Preference
Adult Dosage
Time Course (Onset, Peak, Duration)
Ultra-Short-Acting Adrenergic Bronchodilator Agents
Epinephrine
Adrenalin Chloride
Alpha, beta
SVN: 1% solution (1 : 100), 0.25-0.5 ml (2.5-5.0 mg) 4 times daily
Onset: 3-5 min
Peak: 5-20 min
Duration: 1-3 hr
Primatene Mist
MDI: 0.22 mg/puff, puffs as ordered or needed
Racemic epinephrine
microNefrin, Nephron, S-2
Alpha, beta
SVN: 2.25% solution, 0.25-0.5 ml (5.63-11.25 mg) 4 times daily
Onset: 3-5 min
Peak: 5-20 min
Duration: 0.5-2 hr
Short-Acting Adrenergic Bronchodilator Agents
Metaproterenol
Alupent
Beta-2
SVN: 0.4%, 0.6% solution, tid, qid
Onset: 1-5 min
Tab: 10 mg and 20 mg, tid, qid
Peak: 60 min
Syrup: 10 mg per 5 ml
Duration: 2-6 hr
Albuterol
Proventil HFA, Ventolin HFA, ProAir HFA, AccuNeb, VoSpire ER
Beta-2
SVN: 0.5% solution, 0.5 ml (2.5 mg), 0.63 mg, 1.25 mg and 2.5 mg unit dose, tid, qid
Onset: 15 minPeak: 30-60 minDuration: 5-8 hr
MDI: 90 µg/puff, 2 puffs tid, qid
Tab: 2 mg, 4 mg, and 8 mg, bid, tid, qid
Syrup: 2 mg/5 ml, 1-2 tsp tid, qid
Pirbuterol
Maxair Autohaler
Beta-2
MDI: 200 µg/puff, 2 puffs every 4-6 hr
Onset: 5 min
Peak: 30 min
Duration: 5 hr
Levalbuterol
Xopenex, Xopenex HFA
Beta-2
SVN: 0.31 mg/3 ml 3 times daily, 0.63 mg/3 ml 3 times daily, or 1.25 mg/3 ml 3 times daily, concentrate 1.25 mg/0.5 ml, 3 times daily
Onset: 15 min
Peak: 30-60 min
Duration: 5-8 hr
MDI: 45 µg/puff, 2 puffs every 4-6 hr
Long-Acting Adrenergic Bronchodilator Agents
Salmeterol
Serevent Diskus
Beta-2
DPI: 50 µg/blister twice daily
Onset: 20 min
Peak: 3-5 hr
Duration: 12 hr
Formoterol
Perforomist, Foradil
Beta-2
SVN: 20 µg/2 ml unit dose, bid
Onset: 15 min
DPI: 12 µg/inhalation, bid
Peak: 30-60 min
Foradil Certihaler
Beta-2
DPI: 8.5 µg/inhalation, bid
Duration: 12 hr
Arformoterol
Brovana
Beta-2
SVN: 15 µg/2 ml unit dose, twice daily
Onset: 15 min
Peak: 30-60 min
Duration: 12 hr
Indications for Use
Indication for Short-Acting Agents
Mode of Action and Effects
Adrenergic Bronchodilator Agents
Short-Acting Noncatecholamine Agents
Single-Isomer Beta Agonists
Long-Acting Adrenergic Bronchodilators
Adverse Effects
Assessment of Bronchodilator Therapy
Anticholinergic Bronchodilators
Indications for Use
Drug
Brand Name
Adult Dosage
Time Course (Onset, Peak, Duration)
Ipratropium bromide
Atrovent HFA
HFA MDI: 17 µg/puff, 2 puffs 4 times daily
Onset: 15 min
SVN: 0.02% solution (0.2 mg/ml), 500 µg 3-4 times daily
Peak: 1-2 hr
Nasal spray: 21 µg or 40 µg, 2 sprays per nostril 2-4 times daily (dosage varies)
Duration: 4-6 hr
Ipratropium bromide and albuterol
Combivent
MDI: Ipratropium 18 µg/puff and albuterol 90 µg/puff, 2 puffs 4 times daily
Onset: 15 min
Peak: 1-2 hr
Duration: 4-6 hr
DuoNeb
SVN: Ipratropium 0.5 mg and albuterol 2.5 mg
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access


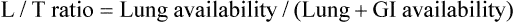
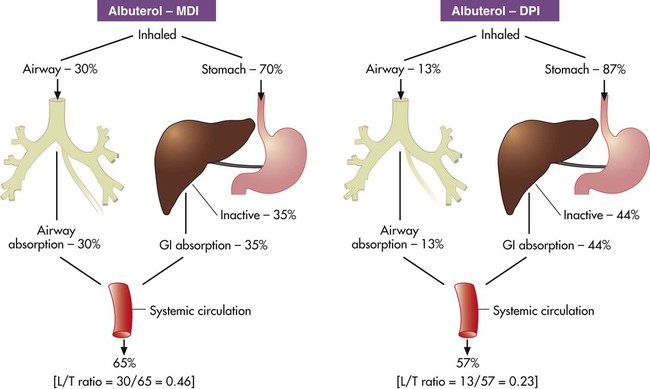
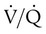 ) ratio (decrease in PaO2/SpO2)
) ratio (decrease in PaO2/SpO2)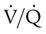 with inhalation of the aerosol is not clinically significant and reverses quickly. Bronchospasm resulting from chlorofluorocarbon propellants can be prevented by changing to newer hydrofluoroalkane-propelled MDIs or a different aerosol delivery form.
with inhalation of the aerosol is not clinically significant and reverses quickly. Bronchospasm resulting from chlorofluorocarbon propellants can be prevented by changing to newer hydrofluoroalkane-propelled MDIs or a different aerosol delivery form.