Adjuvant Chemotherapy for Non-Small-Cell Lung Cancer
Natasha B. Leighl
Frances A. Shepherd
Lung cancer remains the leading cause of cancer mortality in western countries, accounting for more deaths than breast, colon, and prostate cancers combined.38 Although surgical resection remains the treatment of choice for early-stage non-small-cell lung cancer (NSCLC), the 5-year survival rates for stage I and II disease are only 60% to 70% and 35% and 40% respectively.32 The high rates of systemic relapse from lung cancer suggest that despite the apparent early clinical and pathologic stage, micrometastatic disease is present at the time of surgery. For over 30 years, the hypothesis that the risk of distant metastatic spread could be decreased with the use of adjuvant chemotherapy has been tested. Platinum-based chemotherapy regimens, the current standard for the first-line treatment of advanced NSCLC, have been tested against observation alone in randomized trials since the 1970s in North America and Europe, while Japanese investigators have undertaken several trials of oral regimens involving tegafur (an oral 5-fluorouracil derivative) plus uracil (UFT). Numerous randomized controlled trials in the 1980s and 1990s were unable to demonstrate a consistent survival benefit for adjuvant chemotherapy. However, more recently, three pivotal trials have demonstrated the benefit of adjuvant platinum-based systemic chemotherapy in completely resected NSCLC (see Table 116-1). A paradigm shift has resulted, with the establishment of adjuvant platinum-based chemotherapy as a new standard for good performance status patients with resected NSCLC.2,7,61 This chapter describes early adjuvant trials, the current landmark studies of adjuvant therapy, and the focus of current and future research.
Postoperative radiotherapy has also been explored as a means of decreasing the risk of recurrence after NSCLC resection. A meta-analysis including >2,000 patients in nine randomized trials (stages I to III), published in 1998 and recently updated, suggested a negative impact on survival from postoperative radiotherapy in NSCLC.5 Adjuvant radiation treatment was associated with an increase in the relative risk of death of 21% and an absolute decrease in 2-year survival of 7%, from 55% to 48%. Patients with stage I and II disease were more likely to have a detrimental effect. Even in the setting of stage III disease, the meta-analysis data do not support a survival benefit, and evidence of reduced local recurrence risk is conflicting.29 However, other studies continue to suggest potential benefit for adjuvant radiotherapy in N2 or stage III disease. A retrospective analysis of the Surveillance, Epidemiology and End Results (SEER) database identified 7,465 patients with resected stage II or III NSCLC.28 In a subgroup analysis of patients with resected N2 nodal disease, postoperative radiotherapy was associated with improved survival (hazard ratio [HR] = 0.855, 95% confidence interval [CI] 0.762–0.959, p = 0.077). Also, subgroup analysis of the ANITA trial (Adjuvant Navelbine International Trialists Association), a recent pivotal trial of adjuvant third-generation chemotherapy, demonstrated that patients with resected N2 disease who received postoperative radiotherapy had better survival, in both the observation and adjuvant chemotherapy arms (5-year survival 21% versus 17%, observation arm; 47% versus 34%, chemotherapy arm).7 To better evaluate the impact of adjuvant radiation in resected N2 disease using current radiotherapy techniques, the LUNGART trial has been initiated in Europe, randomizing patients to either adjuvant radiation or observation after adjuvant chemotherapy.
Platinum-Based Chemotherapy
Early Studies (Pre-1990)
Adjuvant CAP chemotherapy (cyclophosphamide, doxorubicin, and cisplatin) was evaluated in several studies in resected NSCLC during the 1970s and 1980s. At the same time this regimen was being evaluated in advanced disease, where, despite its modest activity and efficacy, it was found to improve survival in metastatic disease over best supportive care alone.41 Two of the adjuvant trials suggested a potential survival benefit from adjuvant therapy, but the differences were not statistically significant, in part owing to the small sample sizes of these trials.17,33 The Lung Cancer Study Group (LCSG) randomized 141 patients with completely resected stage II and III NSCLC to receive either six cycles of CAP chemotherapy or, in the control arm, bacillus Calmette-Guérin (BCG) plus levamisole immunotherapy (LCSG 772).17 Median and 2-year survival trended to better outcomes in the CAP-treated patients (median 23 versus 16 months; 2-year survival 41% versus 30% control). However, because of the small sample size, the differences were not significant. The second study to suggest a benefit from adjuvant CAP chemotherapy was a Finnish trial of 110 patients with completely resected T1-3N0 NSCLC.33 Patients had a higher 5-year survival rate with the
addition of chemotherapy, 67% versus 56% (p = 0.05). In the larger “confirmatory” study from the LCSG (LCSG 801), Feld et al.9 randomized 283 patients with stage IB and II completely resected NSCLC to receive either four cycles of CAP chemotherapy or observation alone. Only 53% of patients randomized to the CAP arm received all four cycles of planned therapy, and no statistically significant survival difference was observed after a mean follow-up of almost 4 years.
addition of chemotherapy, 67% versus 56% (p = 0.05). In the larger “confirmatory” study from the LCSG (LCSG 801), Feld et al.9 randomized 283 patients with stage IB and II completely resected NSCLC to receive either four cycles of CAP chemotherapy or observation alone. Only 53% of patients randomized to the CAP arm received all four cycles of planned therapy, and no statistically significant survival difference was observed after a mean follow-up of almost 4 years.
Table 116-1 Selected Randomized Trials of Platinum-Based Adjuvant Chemotherapy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Although several meta-analyses of adjuvant trials have appeared in the literature,4,14,15,18,34,46,47 the first and most widely referenced was published by the NSCLC Collaborative Group in 1995, examining the impact of chemotherapy in all stages of disease.34 Individual patient data from 14 randomized trials of surgery with or without chemotherapy, including 4,357 patients, were evaluated, eight using cisplatin-based treatment (n = 1,394) and five using long-term alkylating agents (n = 2,145). For trials using alkylating agents, inferior survival was seen in the chemotherapy group compared with surgery alone, with a hazard ratio of 1.15 (p = 0.005), translating to a 5% absolute decrease in survival at 5 years. In studies of cisplatin-containing regimens, however, adjuvant chemotherapy was associated with a trend toward improved survival over surgery alone, an absolute survival benefit of 5% at 5 years, or a 13% relative reduction in risk of death (HR, 0.87; 95% CI 0.74–1.02; p = 0.08). Seven additional trials with 807 patients compared surgery and radiation with or without chemotherapy, and six trials used cisplatin-based treatment. There was no significant improvement in survival with the addition of chemotherapy to surgery and radiation (HR for all trials, 0.98, p = 0.76; HR 0.94 for cisplatin-based trials; 95% CI 0.79–1.11, p = 0.46).
Based on these data, adjuvant therapy remained an important question for future research studies. Ensuring adequate sample size to power trials sufficiently, finding more active regimens against NSCLC, and enhancing patient compliance, in part through better supportive care, emerged as important considerations in the design of future adjuvant studies.
Regimens Containing Second-Generation Vinca Alkaloids and Epipodophyllotoxins
By the 1990s, there was a shift from the use of alkylating agents, now recognized as potentially detrimental in NSCLC,34 to the use of second-generation chemotherapy agents such as the vinca alklaloids vinblastine and vindesine. Two small trials in Japan compared adjuvant vindesine/cisplatin chemotherapy to observation after complete resection of stage III disease, but neither demonstrated a survival benefit for adjuvant therapy.35,52 The Adjuvant Lung Project Italy (ALPI) trial was a much larger, multicenter study in which 1,196 patients with stages I to IIIA resected NSCLC were randomized to receive three cycles of adjuvant mitomycin, vindesine, and cisplatin (MVP) chemotherapy or observation alone.44 Sixty-nine percent of patients received all three planned cycles of MVP. Adjuvant radiotherapy was permitted at the discretion of the participating centers, and 65% and 82% of patients in the adjuvant therapy and control arms, respectively, received radiation. After a median follow-up of 64.5 months, there was no statistically significant difference in overall or progression-free survival between treatment groups. A small randomized study of adjuvant MVP in patients with completely resected stage I NSCLC also failed to find a significant improvement in survival, but did show an improved disease-free survival for patients in the adjuvant therapy arm.37 At a mean follow-up of 7.3 years, patients treated with adjuvant therapy had 5- and 10-year disease-free survival rates of 88.8% and 76.8%, compared with 64.8% and 54.8% in the observation arm (p = 0.002). However, with only 59 patients in each arm, 5- and 10-year overall survival rates were similar between the groups (81.4% and 65.0% in the chemotherapy arm, 74.6% and 56.3% in the control group, p = 0.19).
The Big Lung Trial was a unique study, designed to assess the impact of chemotherapy when added to differing primary treatments for all stages of NSCLC.60 Of 381 patients with resected stage I–III disease, 192 were randomized to receive chemotherapy. While 97% received adjuvant treatment, 3% received preoperative induction. Between 11% and 15% of patients had an incomplete resection, and 14% of patients received radiotherapy. MVP was administered to 42% of patients, MIC (mitomycin/ifosfamide/cisplatin) to 33%, vindesine/cisplatin to 3%, and vinorelbine/cisplatin to 22%. At a median follow-up of 34.6 months, there was no improvement in either overall or progression-free survival, with hazard ratios of 1.02 (95% CI 0.77–1.35, p = 0.90) and 0.97 (95% CI 0.74–1.26, p = 0.81), respectively.
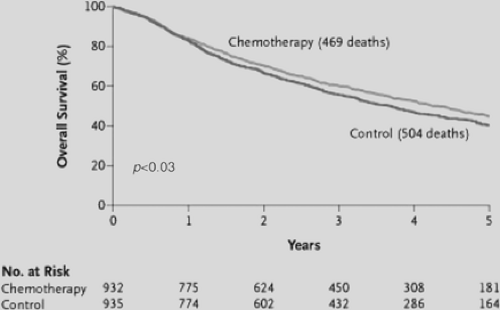
The International Adjuvant Lung Cancer Trial (IALT) is the largest multicenter randomized trial of adjuvant chemotherapy reported to date and was the first pivotal trial to demonstrate significantly improved survival with the addition of adjuvant chemotherapy in resected NSCLC.2 A total of 1,867 patients with stages I–III completely resected NSCLC were randomized to chemotherapy or observation. To facilitate accrual to the study, stage of disease, cisplatin dose, chemotherapy regimen, and use of adjuvant radiation were permitted to vary among centers. At a median follow-up of 56 months, overall survival was longer in the adjuvant chemotherapy arm, with an absolute 5-year survival improvement of 4.1%, and 14% relative reduction in the risk of death (HR, 0.86; 95% CI 0.76–0.98, p < 0.03), shown in Figure 116-1. This improvement in survival with chemotherapy was maintained even when adjusted for use of radiation, different chemotherapy regimens, total cisplatin dose, or disease stage. Etoposide/cisplatin was administered to 56.5% of patients, only 26.7% received the third-generation combination vinorelbine/cisplatin, and 25% of patients received adjuvant radiation. The magnitude of survival benefit seen in the IALT study is similar to the 5% absolute survival improvement at 5 years demonstrated in the NSCLC Collaborative group meta-analysis,34 although the latter improvement was not statistically significant. Despite this, IALT is considered to be the first of three pivotal trials to establish significant benefit from adjuvant chemotherapy in resected NSCLC.
However, the use of adjuvant chemotherapy was not widely adopted despite the statistically significant results of the IALT trial.20 This may be attributed to the negative results of other recently reported trials—such as ALPI,44 the Big Lung Trial,60 and ECOG trials22—as well as the modest survival gain of 4% at 5 years, use of second-generation rather than third-generation chemotherapy, and associated treatment toxicity.
Trials of Adjuvant Chemotherapy Plus Radiation
Although many patients in adjuvant chemotherapy trials have also received radiotherapy, there is no evidence at present that combining adjuvant chemotherapy and radiation is of benefit
in patients with completely resected NSCLC. Three studies comparing postoperative chemotherapy plus radiation with postoperative radiation alone failed to demonstrate a benefit from the combination.6,22,27 The LCSG 791 trial enrolled patients with incompletely resected NSCLC, defined as either a positive resection margin or microscopic involvement by tumor in the highest lymph node sampled.27 Patients received postoperative radiation followed by six cycles of CAP chemotherapy or observation alone. Although recurrence-free survival was better in the chemotherapy arm (p = 0.004), there was no difference in overall survival between the two groups.
in patients with completely resected NSCLC. Three studies comparing postoperative chemotherapy plus radiation with postoperative radiation alone failed to demonstrate a benefit from the combination.6,22,27 The LCSG 791 trial enrolled patients with incompletely resected NSCLC, defined as either a positive resection margin or microscopic involvement by tumor in the highest lymph node sampled.27 Patients received postoperative radiation followed by six cycles of CAP chemotherapy or observation alone. Although recurrence-free survival was better in the chemotherapy arm (p = 0.004), there was no difference in overall survival between the two groups.
A French Cooperative Group study randomized patients with completely resected stages I to III NSCLC to receive either radiotherapy alone or radiation followed by chemotherapy with cyclophosphamide/doxorubicin/cisplatin/vincristine/lomu- stine.6 The addition of chemotherapy did not improve either disease-free or overall survival compared with adjuvant radio- therapy alone.
Despite these results and those of the PORT meta-analysis,5 adjuvant radiation remained popular as a means of reducing local recurrence rates.22 The Eastern Cooperative Oncology Group (ECOG) randomized 488 patients to receive postoperative adjuvant radiotherapy with 50.4 Gy in 28 daily fractions, or the same radiation dose plus concurrent etoposide/cisplatin chemotherapy for four cycles.22 At a median follow-up of 44 months, survival was comparable for both treatment groups (39 and 38 months median survival for radiation and concurrent arms respectively), and rates of local control were also similar. Thus the value of adjuvant chemotherapy concurrent with radiation remains a question for future trials in the setting of completely resected NSCLC. It is important to note that radiotherapy in the setting of incomplete resections (e.g., positive margins, residual disease) remains an appropriate consideration.
Third-Generation Chemotherapy Regimens
Since 2004, three important trials of adjuvant therapy using third-generation chemotherapy regimens, currently the most active in NSCLC, have been presented (summarized in Table 116-2).7,50,61 At least two of these trials have been pivotal in establishing the role of adjuvant chemotherapy after complete resection of NSCLC as a new standard of care.
The Cancer and Leukemia Group B (CALGB) conducted a randomized study of adjuvant paclitaxel/carboplatin for four cycles versus observation in patients with stage 1B (T2N0M0) NSCLC.50 The original accrual target for CALGB 9633 was 500 patients, but this was decreased to 384 owing to slow accrual. The data safety monitoring board recommended early closure after accrual of 344 patients because of a significant survival difference seen in a planned interim analysis. A preliminary presentation, based on 57% of events required for final analysis and only 34 months median follow-up, suggested a 12% absolute improvement in survival at 4 years with the addition of chemotherapy, 71% versus 59% (HR 0.62; 95% CI 0.41–0.95; p = 0.028). Initially, this led to widespread uptake of treatment with this regimen in the United States in the setting of resected IB NSCLC. However, with longer follow-up at a median of 57 months, reanalysis of these data 2 years later suggested only a trend to improved survival (HR 0.80, 95% CI 0.60–1.07, p = 0.10), although better survival was still seen in the adjuvant chemotherapy arm at 2 and 3 years (90% versus 84% at 2 years, p = 0.05; 79% versus 71% at 3 years, p = 0.043). Disease-free survival remained significantly improved with the addition of chemotherapy (89 months versus 52 months median failure-free survival; HR 0.74, 95% CI 0.47–0.96, p = 0.03). Treatment was extremely well tolerated, with no toxic deaths, and 85% of patients completed all four planned cycles of therapy. Although the updated analysis suggested the study was negative, unplanned subset analysis identified that patients with tumors ≥4 cm may still derive a survival benefit, with a hazard ratio of 0.66 (p = 0.04). The question remains as to whether this study is truly negative or merely underpowered to detect a small difference in outcome. This study reinforces the need for larger trials in the IB subgroup of patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree