Adenoid Cystic Carcinoma and Other Primary Salivary Gland–Type Tumors of the Lung
Richard S. D’Agostino
The submucosal serous and mucous glands of the tracheobronchial tree are similar to those of the major and minor salivary glands and can give rise to morphologically and biologically analogous tumors. Adenoid cystic carcinoma, mucoepidermoid carcinoma, mixed tumors of salivary gland type, acinic cell carcinoma, and oncocytoma have in prior years been included with carcinoid tumors under the misleading and antiquated rubric bronchial adenomas. In contrast, true adenomas—adenoid cystic carcinoma, mucoepidermoid carcinoma, and acinic cell carcinoma—are malignant lesions. Although their growth rate is often indolent, local invasion and lymph node metastases ultimately may occur, and in some cases biological behavior is aggressive. Mixed tumors and oncocytoma can behave in a benign or malignant fashion. These noncarcinoid, salivary gland–type tumors of the lung are uncommon and constitute less than 1% of all primary lung neoplasms.
Adenoid Cystic Carcinoma
Adenoid cystic carcinoma, formerly called cylindroma, most often arises in the salivary glands. Less frequent primary sites include the lung, breast, skin, uterine cervix, esophagus, and prostate.
Pathology
The majority of pulmonary adenoid cystic carcinomas develop centrally in the trachea and major bronchi. These otherwise uncommon tumors account for 25% to 50% of all primary tracheal malignancies. Dalton and Gatling12 and Inoue and colleagues,26 among others, reported instances of primary adenoid cystic carcinoma arising peripherally and suggested that this may be the case in approximately 10% of patients. Peripheral lesions, however, are more likely to represent metastases from an extrathoracic primary site. Synchronous hematogenous metastases at the time of initial presentation are rarely encountered. However, Maziak and coworkers33 and Prommegger and Salzer45 noted late metachronous hematogenous metastases in 44% and 55% of their patients, respectively. On macroscopic inspection, adenoid cystic carcinoma appears white, pink, or light tan and is either polypoid or annular. The overlying mucosa is usually intact, although ulceration may occur. Direct transluminal extension is the rule. Malignant submucosal and perineural infiltration is common and often extends a considerable distance proximally and distally from the main tumor mass (Fig. 123-1).
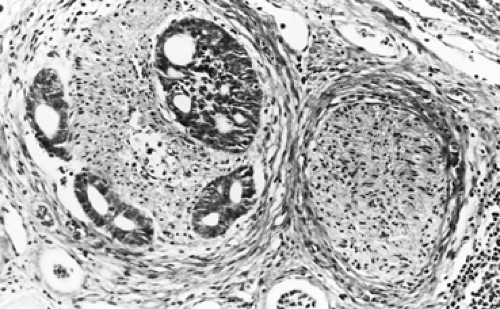
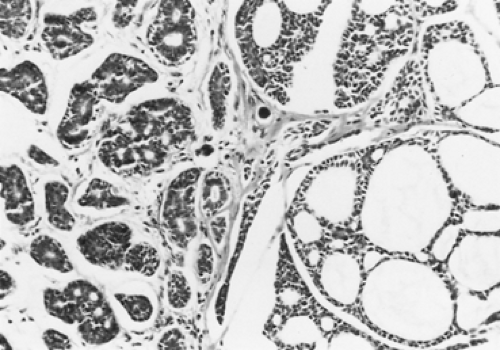
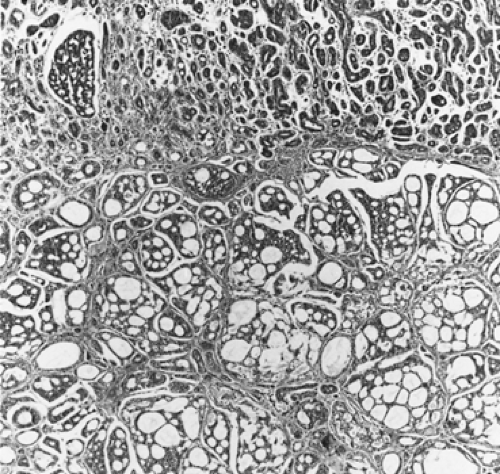
Adenoid cystic carcinomas are histologically similar regardless of their primary site. The following three patterns are recognized: cribriform, tubular, and solid. The cribriform or cylindromatous subtype is the classic finding (Fig. 123-2). Nests and columns of small rounded or polyhedral cells arranged in a sieve-like pattern surround gland-like spaces filled with periodic acid–Schiff–positive material. The tubular pattern is defined by cords of polygonal cells containing central ducts that are separated from surrounding stroma. The solid or basaloid pattern is morphologically the least differentiated and consists of sheets of small cuboidal cells with prominent mitoses and occasional small clusters of larger polygonal cells. An individual tumor can display more than one pattern (Fig. 123-3). Nomori and associates39 found that tumors with solid elements are associated with an aggressive clinical course and with the appearance of distant metastases more often than with other patterns. However, Albers and coworkers1 found histologic grade to be an inconclusive prognostic factor. Moran35 noted that the immunohistochemical profile of adenoid cystic carcinoma is dominated by the myoepithelial cell proliferation. These tumor cells showed positive staining with both wide-spectrum and low-molecular-weight keratin as well as with vimentin and actin antibodies. Reactivity to S-100 protein has been variable. Staining for glial fibrillary acidic protein was uniformly negative. This latter finding can be used to distinguish adenoid cystic carcinoma from pleomorphic adenoma, a tumor that also has a prominent myoepithelial cell component. Although the histopathologic features of adenoid cystic carcinoma are usually distinctive enough to allow for definitive diagnosis by routine light microscopy, this tumor can be confused with adenocarcinoma, especially when faced with a small biopsy specimen. In such situations, the demonstration of a myoepithelial cell immunophenotype supports the diagnosis
of adenoid cystic carcinoma. Lin and coworkers32 found 8 of 9 patients with tracheobronchial adenoid cystic carcinoma negative for the biomarkers p53, HER-2/neu, and COX-2. The one patient positive for HER-2/neu developed distant metastases 4 years postoperatively. Albers and colleagues1 noted all 13 tumors in their series to stain positively for c-kit protein (CD117, a type III tyrosine kinase receptor) but could find no correlation between histologic grade and Ki-67 (a marker for cell proliferation) activity.
of adenoid cystic carcinoma. Lin and coworkers32 found 8 of 9 patients with tracheobronchial adenoid cystic carcinoma negative for the biomarkers p53, HER-2/neu, and COX-2. The one patient positive for HER-2/neu developed distant metastases 4 years postoperatively. Albers and colleagues1 noted all 13 tumors in their series to stain positively for c-kit protein (CD117, a type III tyrosine kinase receptor) but could find no correlation between histologic grade and Ki-67 (a marker for cell proliferation) activity.
Clinical Features
Adenoid cystic carcinoma typically occurs in patients 40 to 50 years of age, but it has been reported in persons ranging in age from 18 to 82 years. Men and women are affected equally. There is no known association with tobacco use or environmental toxins. Because they are located centrally and are often not apparent on chest radiographs, most tumors are detected only after they produce symptoms. Patients generally describe one or more of the following problems: dyspnea, cough, hemoptysis, wheezing, hoarseness, stridor, or recurrent pneumonias. In some cases, significant tracheal or main bronchial obstruction occurs early and can be life threatening. However, in many instances the intraluminal component is relatively small despite extensive submucosal and extraluminal spread. This pattern, coupled with typically slow growth, can produce an insidious presentation. An interval of several months to years may elapse between the onset of symptoms and the establishment of a diagnosis. Gaissert and associates19 noted mean symptom duration of 24 months prior to diagnosis. Some patients are treated for prolonged periods for an erroneous diagnosis of asthma or chronic obstructive lung disease. The physical examination may demonstrate diminished or absent breath sounds because of complete obstruction of a lobe or an entire lung, a unilateral wheeze from partial blockage of a main bronchus, or stridor produced by tracheal involvement, but also may be negative. Peripheral adenopathy and clubbing are not features of adenoid cystic carcinoma.
Radiographic Features
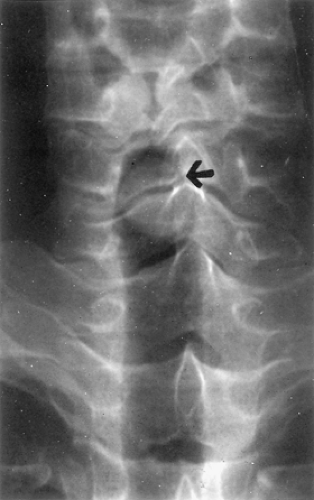
Owing to the central location of most tumors, the chest radiograph may appear normal, but can show atelectasis of a lobe or an entire lung. A hilar or mediastinal mass may be present. Subtle changes in airway contour occasionally are noted, particularly with lesions of the intrathoracic trachea. On the other hand, tumor masses in the cervical trachea are often easily appreciated on plain neck radiographs (Fig. 123-4). Standard tomography has been replaced by computed tomography (CT), which accurately defines both intraluminal and transverse extraluminal tumor and is helpful in detecting metastases to the lung or pleura. However, conventional CT scan usually underestimates the longitudinal extent of airway involvement because of partial volume averaging and the tendency for submucosal tumor extension. Shanley50 and Akata2 and their colleagues found magnetic resonance imaging, with its ability to image multiple anatomic planes and to provide variably weighted images,
superior to conventional CT for delineating the extent of both submucosal infiltration and mediastinal involvement. However, Kwak and colleagues,30 among others, have demonstrated that the newer technique of helical CT scan with multiplanar image reconstruction allows for rapid image acquisition and excels in defining both the longitudinal and lateral extent of tumor. Nevertheless, helical CT is a poor predictor of perineural and mediastinal fat invasion. Campistron and associates6 have reported a singular case of adenoid cystic carcinoma arising in the bronchus intermedius, with a synchronous histologically proven metastasis to the liver, detected by positron emission tomography (18FDG PET-CT). In some cases, contrast esophograms may be helpful by showing esophageal compression or invasion.
superior to conventional CT for delineating the extent of both submucosal infiltration and mediastinal involvement. However, Kwak and colleagues,30 among others, have demonstrated that the newer technique of helical CT scan with multiplanar image reconstruction allows for rapid image acquisition and excels in defining both the longitudinal and lateral extent of tumor. Nevertheless, helical CT is a poor predictor of perineural and mediastinal fat invasion. Campistron and associates6 have reported a singular case of adenoid cystic carcinoma arising in the bronchus intermedius, with a synchronous histologically proven metastasis to the liver, detected by positron emission tomography (18FDG PET-CT). In some cases, contrast esophograms may be helpful by showing esophageal compression or invasion.
Diagnosis
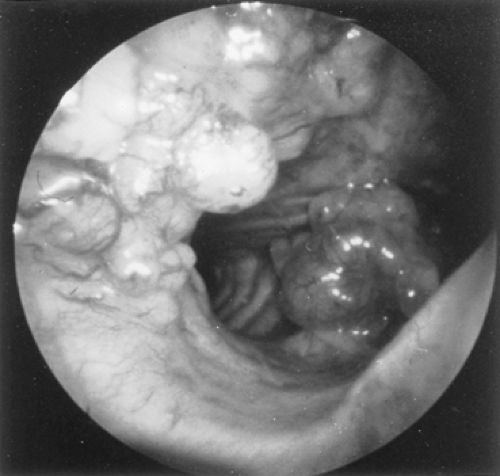
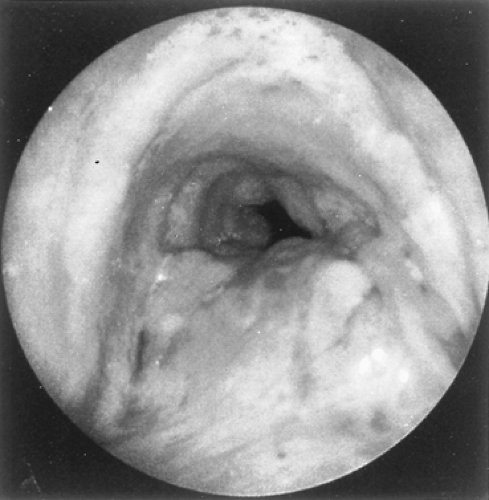
Because adenoid cystic carcinomas usually are covered by normal bronchial epithelium, cytologic examination of the sputum is generally unrewarding unless the overlying respiratory epithelium has been eroded. Except for the unusual circumstance of a peripherally situated lesion, these tumors are identified at bronchoscopy, and the diagnosis is established by transmucosal biopsy. The typical bronchoscopic appearance is that of a broad-based polypoidal mass partially or completely obstructing the airway (Fig. 123-5). Alternatively, the tumor may appear as a diffuse, submucosal infiltration causing luminal distortion and obstruction (Fig. 123-6). Although Attar and associates,4 among others, reported substantial bleeding after biopsy of bronchial tumors in some patients, hemorrhage appears to be more of a problem with carcinoid and mucoepidermoid tumors than with adenoid cystic carcinomas. Care must be taken with large intraluminal tumors as instrumentation may lead to acute airway compromise from swelling, bleeding or secretions. The rigid bronchoscope provides a greater degree of control in such instances and also can be used to core out obstructing tumors. These situations are best managed under a general anesthetic and in close coordination with the anesthesiologist. The extent
of tracheal involvement should be thoroughly assessed both visually and with transmucosal biopsies to determine the length of trachea available for reconstruction.
of tracheal involvement should be thoroughly assessed both visually and with transmucosal biopsies to determine the length of trachea available for reconstruction.
Treatment
The treatment of choice is resection when feasible. Airway stents and radiation therapy should not be employed as initial therapeutic maneuvers in otherwise resectable patients. Gaissert and colleagues19 were able to perform resection in 75% of their patients. Contraindications to resection were most often due to the length of airway involvement and to the extent of regional disease. It cannot be overemphasized that submucosal and perineural infiltration commonly extends beyond macroscopic tumor boundaries. Intraoperative frozen section examination of resection margins should be performed routinely as a method of ensuring adequate tumor clearance. Conlan and associates,10 Grillo and Mathisen,21 and Gaissert and coworkers19 found that tumor spread to hilar and mediastinal lymph nodes does not preclude long-term survival. In the case of tracheal resections, Grillo and Mathisen21 stressed that lymph nodes adjacent to the tumor should be resected and distant nodes sampled, but that extensive dissection should be avoided because it may jeopardize anastomotic healing. They also pointed out that, in some cases, one must accept a microscopically positive margin rather than extend the resection and produce tension on the suture line. Resection should not be performed when it would result in grossly positive peritracheal margins or when the length of tracheal involvement prevents safe reconstruction. Tumors involving a lobar bronchus can often be managed with lobectomy, using bronchoplastic techniques where applicable. However, submucosal spread in the main-stem bronchus may mandate a pneumonectomy. Extensive tracheal or carinal involvement presents more complex situations. Gaissert and colleagues19 noted carinal involvement in 41 of 101 patients with an associated perioperative mortality of 16% for carinal resection during the 40-year span of their study. Although perioperative mortality has decreased in recent years, the risks of resection must be considered carefully because treatment methods other than resection can provide effective palliation for long periods. Grillo and Mathisen,21 Pearson and associates,41 and Perelman and coworkers43 described their extensive experience with these situations. The techniques of bronchial sleeve lobectomy and tracheal and carinal resections are discussed in other chapters.
Most adenoid cystic carcinomas are radiosensitive and radiation therapy is the primary treatment for extensive inoperable cancers and for patients in whom resection carries an unacceptable risk. Fields and colleagues18 noted that in patients treated with external beam radiation therapy alone, the rate of complete response was significantly related to a tumor dose of 60 Gy or more; currently tumor doses of between 55 and 65 Gy are commonly employed. Endoluminal high dose-rate brachytherapy is another method that appears to have a palliative role. Harms and associates23 and Carvalho and colleagues,8 among others, have reported successful primary tumor control with high dose-rate brachytherapy as a supplement to external beam radiotherapy, although late tracheitis and stenosis necessitated tracheal stent in some of their patients. Given the surgically close or microscopically positive margins for many resected patients, external beam radiation therapy is now utilized routinely as an adjunct to resection. Gaissert and associates,19 among others, have employed doses between 45 and 65 Gy beginning 6 to 8 weeks following resection. Although limited experience with preoperative neoadjuvant radiation therapy has been reported by Pearson and colleagues41 and Perelman and Koroleva42 in the past, it is generally avoided in the current era due to concerns over the impaired healing of radiated tissue.
For many patients with unresectable or recurrent disease, tumor debulking by neodymium:yttrium-aluminum-garnet (Nd:YAG) laser ablation may provide satisfactory palliation. Diaz-Jimenez and colleagues14 reported laser treatment in five patients with unresectable adenoid cystic carcinoma causing tracheal and mainstem bronchial obstructions of 75% to 90% of luminal diameter. One to two laser applications provided palliation for up to 33 months in four cases. The remaining patient was treated on multiple occasions over the course of 49 months before dying of hemoptysis. Treatment may be affected with either the fiberoptic or rigid bronchoscope, although the latter is favored by most clinicians because it allows easier tumor debulking by morcellation, rapid control of hemorrhage, and protection of the contralateral airway if so needed. As reported by Personne44 and Albers1 and their colleagues, laser photocoagulation and radiation therapy are often used in combination for the palliation of symptoms related to adenoid cystic carcinoma and can be associated with prolonged survival. Laser ablation should be considered before radiation therapy in patients with critical proximal airway obstruction because irradiation in this setting may produce enough edema to cause asphyxia. There are no published data regarding the efficacy of systemic chemotherapy for pulmonary adenoid cystic carcinoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree