Acute Respiratory Failure in the Surgical Patient
INTRODUCTION
Advances in surgical technique, anesthesia and analgesia, and postoperative supportive care have emboldened surgeons to consider an expanding spectrum of patients for surgical interventions. In most instances, the success or failure of the surgery is defined not in the operating room, but postoperatively, when the adverse effects of surgery may first become apparent and when intercurrent complications may jeopardize the patient’s recovery. The respiratory system is particularly vulnerable to the effects of general anesthesia and surgery, and postoperative respiratory impairment is common. While generally mild and well tolerated in otherwise healthy, young patients, postoperative respiratory compromise may have serious consequences in the elderly and in patients with pre-existing lung disease. A number of postoperative complications, such as pneumonia, aspiration pneumonitis, and acute respiratory distress syndrome (ARDS) may lead to respiratory compromise independent of the patient’s presurgical status.
This chapter focuses on the most serious consequence of perioperative respiratory compromise—acute respiratory failure. This complication is associated with a 30-day mortality rate in the range of 25% following major surgical procedures, compared to approximately 1% for unaffected patients.1 In addition to its adverse impact on survival, respiratory failure prolongs intensive care and hospital stay, delays convalescence, and increases healthcare costs among survivors. Clinicians who provide preoperative evaluation and postoperative care must be able to identify high-risk patients who require a greater degree of vigilance, and to rapidly recognize and appropriately treat the complications that result in postoperative respiratory failure.
IDENTIFICATION OF THE HIGH-RISK PATIENT
There have been several published studies using large databases that have provided insight into the incidence of postoperative respiratory failure, its impact on survival, and factors associated with increased risk. In one of the earliest surveys involving over 7000 patients undergoing various gastrointestinal, urological, gynecological, and orthopedic procedures, respiratory failure requiring mechanical ventilation beyond 24 hours occurred in only 0.8%.2 More recently, analysis of a database of 180,359 patients undergoing major general or vascular surgical procedures at 128 Veterans Affairs hospitals and 14 private sector hospitals documented a 3% incidence of postoperative respiratory failure (defined as mechanical ventilation beyond 48 hours after surgery or need for reintubation).1 Thirty-day mortality was 27% for the group with respiratory failure compared to only 1.4% for those without. Twenty-eight variables were identified that were independently associated with increased risk. These included higher American Society of Anesthesiologists (ASA) class, preoperative sepsis, emergency as opposed to elective procedure, impaired preoperative renal function, history of smoking or COPD, and older age. The type of procedure also impacted risk (Table 104-1), with the greatest incidence of respiratory failure associated with upper aerodigestive tract surgery, thoracic or thoracoabdominal aneurysm repair, thoracic surgery, and gastrointestinal and hepatobiliary surgery. The investigators incorporated the 28 variables (Table 104-2) into a respiratory risk index (Table 104-3) to predict the likelihood that a patient will develop respiratory failure and validated it using a second patient cohort. Unfortunately, the complexity of the model renders it unwieldy and has limited its widespread acceptance.
TABLE 104-1 Incidence of Respiratory Failure Following Various Surgical Procedures
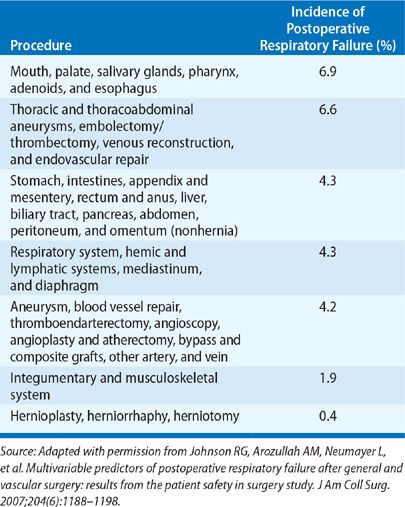
TABLE 104-2 Scoring System for Estimating Risk of Postoperative Respiratory Failure: Parameters Used and Score Assigned
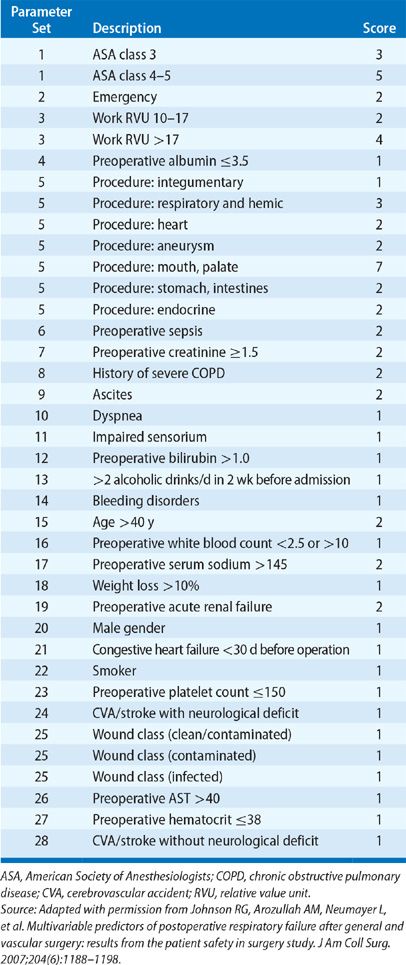
TABLE 104-3 Scoring System for Estimating Risk of Postoperative Respiratory Failure: Respiratory Risk Index
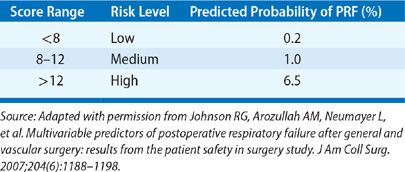
In the largest study of this nature to date, investigators used the 2007 American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database of over 210,000 patients who underwent a broad array of surgical procedures to identify risk factors and derive a predictive model for assessing risk of postoperative respiratory failure.3 Respiratory failure developed in 3.1% of patients, with an associated 30-day mortality rate of 26% compared to 0.98% for patients not experiencing this complication. On multivariate logistic regression analysis, five preoperative predictors of postoperative respiratory failure were identified: type of surgery (highest risk with brain, abdominal, aortic surgery), emergency cases, poor preoperative functional status, preoperative sepsis, and higher ASA class. These variables were incorporated into a predictive model that was validated in a second cohort of over 250,000 patients from the 2008 NSQIP database. An interactive risk calculator, available online at http://www.surgicalriskcalculator.com/prf-risk-calculator, was then developed that estimates the likelihood of respiratory failure for a given patient.
Pulmonary consultants providing preoperative assessment are commonly asked to specifically comment on the risk posed by a patient’s history of COPD. COPD has been identified as an independent predictor of postoperative respiratory failure in numerous studies.1,4–7 The largest and most comprehensive study utilized the NSQIP database containing over 460,000 patients who underwent a variety of surgical procedures. COPD was independently associated with an increased risk of ventilator dependence for more than 48 hours following surgery (odds ratio 1.45) and reintubation (odds ratio 1.54). Notably, neither this study nor the others referenced earlier categorized patients according to preoperative pulmonary function parameters, precluding conclusions about the relationship between severity of COPD and risk of postoperative respiratory failure.
The use of preoperative pulmonary function testing to assess risk of postoperative respiratory failure in COPD patients has been most closely examined in the context of lung resection surgery for lung cancer. Studies of patients undergoing standard thoracotomy and either pneumonectomy or lobectomy have used preoperative spirometry in conjunction with quantitative ventilation/perfusion lung scans to estimate postresection FEV1 and diffusing capacity. While the study populations are small, published case series suggest that a predicted postresection FEV1 or diffusing capacity less than 40% of normal is associated with a high risk for postoperative respiratory failure and death.8–11 In one study that examined the performance characteristics of these parameters, the probability of respiratory failure or death was 33% for those patients with an FEV1 <40% while the probability of avoiding these complications was 90% for those with an FEV1 ≥40%.10 Coupling pulmonary function testing with cardiopulmonary exercise testing allows for more precise risk stratification. Those patient with peak oxygen consumption of ≥10 to 15 mL/kg/min despite predicted postresection FEV1 or diffusing capacity <40% appear to have a low risk of respiratory failure and death following thoracotomy and definitive lung resection procedures.9,12 The use of video-assisted thoracoscopic surgery (VATS) in combination with lobectomy, segmentectomy, or wedge resection appears to be well tolerated by patients with severe lung disease, with only a 4% incidence of respiratory failure and a 1% mortality rate documented in one study of 100 consecutive patients with an FEV1 <35% predicted.13
Studies using preoperative spirometry to assess severity of COPD in patients undergoing cardiac surgery have provided conflicting results with respect to the risk of respiratory failure. One study found that an FEV1 <70% was an independent predictor of post-CABG respiratory failure but the rather broad nature of the spirometric threshold used limits the utility of this finding.4 A study of 1412 patients found that the combined incidence of prolonged postoperative mechanical ventilation (>48 hours) and reintubation increased with increasing severity of COPD: 2.3% in patients without COPD compared to 0%, 13%, and 77% in patients with FEV1 60% to 80%, 40% to 59%, and <40%, respectively. In contrast, the largest study to date utilizing a database of over 11,000 patients did not detect an association between COPD severity and risk of prolonged postoperative mechanical ventilation even among patients with the most severe disease (FEV1 <50%), though there was an increased risk of early mortality in this group.14
Data related to the use of preoperative pulmonary function parameters to stratify risk of postoperative respiratory failure in relation to noncardiothoracic surgical procedures are scant and conflicting. These data are summarized in a recent guidelines statement issued by the American College of Physicians, which concludes that for noncardiothoracic surgery, “insufficient evidence supports preoperative spirometry as a tool to stratify risk.”15 The guideline authors caution that available studies do not define a spirometric threshold below which the risk for noncardiothoracic surgery is prohibitive.
MEASURES TO REDUCE RISK
A number of the factors associated with an increased risk of postoperative respiratory failure can potentially be addressed to mitigate the risk. In some cases, the surgical approach can be modified. For example, use of a transverse abdominal incision appears to carry less risk than a vertical midline incision. Cholecystectomy performed by laparoscopic technique is associated with significantly less compromise in postoperative FEV1 and FVC and more rapid recovery of lung function compared with the conventional open approach.16 For thoracic procedures, median sternotomy and muscle-sparing lateral thoracotomy are better tolerated than posterolateral thoracotomy. However, these approaches provide more limited access to the thorax than does the standard thoracotomy incision and they are generally inadequate for resection of the left lower lobe or for tumors involving the posterior chest wall, diaphragm, or superior sulcus. In addition, removal of bulky tumors via the muscle-sparing approach may be problematic. VATS offers a minimally invasive alternative that is associated with a less severe decrement in respiratory muscle strength and lung function in the immediate perioperative period compared to thoracotomy.17 A propensity-matched analysis of patients undergoing lobectomy by means of VATS versus open thoracotomy demonstrated a significantly lower rate of postoperative reintubation in the VATS cohort (1.4% vs. 3.1%).
Patients with COPD scheduled for surgery should undergo a preparatory pulmonary regimen intended to optimize lung function and minimize airway secretions. This regimen should include smoking cessation, institution or intensification of inhaled bronchodilator and inhaled corticosteroid therapy, and use of oral antibiotics in the presence of purulent secretions or a “loose” cough. Patients should be instructed on the use of incentive spirometry or cough and deep breathing techniques prior to surgery. A short course of oral corticosteroids should be considered in patients who have a significant bronchospastic component to their disease. Other than the assurance of strict compliance with the regimen, there is no reason to believe that hospitalization is superior to outpatient preparation of the patient.
Smoking has been shown to be a risk factor for postoperative pulmonary complications in general and for prolonged ventilatory support in particular.1 Smoking does not appear simply to be a surrogate marker of COPD; rather it poses risk that is independent of the magnitude of pulmonary impairment. Detrimental effects of smoking include bronchial irritation with resultant excessive airway secretions, impairment in mucociliary clearance, and elevation of carboxyhemoglobin levels with consequent impairment in oxygen uptake and tissue oxygen utilization. Observational studies suggest that preoperative smoking cessation is associated with a reduction in pulmonary complications but these studies do not address the specific question of whether abstinence reduces the risk of respiratory failure.18 A prospective, randomized trial investigated the impact of a preoperative smoking cessation program (counseling and nicotine replacement patch) on a number of postoperative complications including respiratory failure, following elective hip and knee replacement. Complete abstinence from smoking was achieved in 60% of the patients in the intervention arm compared to only 7% in the control arm. Despite this marked difference, only one patient in each group experienced postoperative respiratory failure. The obvious limitation of this trial relates to the choice of a surgical population at minimal risk for the complication under investigation. Pending additional studies, it would appear prudent to initiate smoking cessation measures in preparing patients for surgery, though the full benefit with respect to global reduction in pulmonary complications may require a minimum of 8 weeks of abstinence.19
IMPACT OF ANESTHESIA AND POSTOPERATIVE ANALGESIA ON PULMONARY FUNCTION
The potential impact of anesthesia and postoperative analgesia on pulmonary function is related to the form of anesthesia or analgesia employed.
 GENERAL ANESTHESIA
GENERAL ANESTHESIA
Use of general anesthetic agents is associated with a number of well-characterized alterations in pulmonary mechanics, gas exchange, and respiratory drive.20,21 In the controlled environment of the operating room, these physiological derangements are clinically inconsequential and easily overcome by simple adjustments of the ventilator. However, lingering effects of general anesthesia after completion of surgery may impede efforts to extubate the patient or may precipitate respiratory failure in the recovery room.
Administration of general anesthesia, whether by the inhaled or intravenous route, results in an almost immediate loss of diaphragmatic and intercostal muscle tone, a cephalad shift of the diaphragm, and a decrease in the transverse thoracic diameter. These dimensional alterations in thoracic volume result in a 20% reduction in functional residual capacity (FRC) and in the development of compressive atelectasis. As demonstrated using computerized tomography during and after general anesthesia, patients develop crescent-shaped areas of atelectasis in dependent areas of lung within 10 minutes of induction.22 Atelectatic areas comprise approximately 2% to 10% of total lung volume and disappear with the application of positive end-expiratory pressure (PEEP). Dependent atelectasis develops after administration of either inhalational or intravenous anesthetics. A notable exception is ketamine, a drug that is unique in its maintenance of respiratory muscle tone. The degree of atelectasis appears unaffected by whether the patient is breathing spontaneously or is mechanically ventilated.
Areas of dependent atelectasis perturb the normal balance of ventilation and perfusion in the lung. Persistent perfusion of nonventilated atelectatic areas results in an increase in the shunt fraction, which may approach 15%. The magnitude of shunt correlates directly with the volume of atelectatic lung and may be further magnified by impairment of hypoxic pulmonary vasoconstriction induced by certain inhalational anesthetics. Elderly patients, those who are obese, and patients with underlying COPD are most likely to develop clinically apparent hypoxemia in response to general anesthesia; the effect may persist into the early postoperative period.
The inhaled anesthetic agents in common usage are respiratory depressants that blunt the response to both hypoxemia and hypercapnia. These agents depress the ventilatory response to CO2 in a dose-dependent fashion. They have a negligible effect on the hypercapnic response at the low concentrations encountered during emergence from anesthesia. In contrast, hypoxemic drive is markedly attenuated even at very low, subanesthetic concentrations of the volatile agents. As a result of deposition of these agents in muscle and fat, concentrations sufficient to depress hypoxic drive persist for several hours after termination of anesthesia. This can result in significant postoperative respiratory depression in patients with chronic hypercapnia who are dependent on hypoxic ventilatory drive to breathe.
 NEURAXIAL ANESTHESIA
NEURAXIAL ANESTHESIA
It is common practice for those providing preoperative assessment of high-risk patients to recommend the use of neuraxial (i.e., spinal or epidural) anesthesia, predicated on the impression that this route of administration lessens the adverse impact of anesthesia on the respiratory system. Neuraxial anesthesia does possess a number of favorable physiological features. In contrast to the effects of general anesthesia, neuraxial anesthesia preserves diaphragmatic innervation and function. External intercostal muscle paralysis is induced by thoracic levels of neuraxial anesthesia, but the level is generally two dermatomes below the sensory level because of the lesser sensitivity of motor neurons to the effects of the anesthetic agent. Hypoxic pulmonary vasoconstriction is unaffected by neuraxial anesthesia, and the ventilatory response to CO2 is unimpaired; indeed the CO2 response may be heightened. A meta-analysis of trials comparing general to neuraxial anesthesia detected a significant reduction in 30-day mortality, venous thromboembolism, pneumonia, and respiratory depression.23 Another meta-analysis restricted to patients undergoing hip fracture surgery also detected a reduction in 30-day mortality as well as a trend toward a lower incidence of postoperative hypoxemia associated with neuraxial anesthesia.24 While these analyses suggest significant advantages associated with use of neuraxial anesthesia, they have been criticized because of the heterogeneous nature of the surgical populations and the use of older anesthetic techniques and older anesthetic agents.25 Pending further studies, neuraxial anesthesia should not necessarily be viewed as the strategy of choice in more tenuous surgical candidates.
 POSTOPERATIVE ANALGESIA
POSTOPERATIVE ANALGESIA
Postoperative analgesia is an essential component of the care of the surgical patient. Analgesia is important not only in ensuring patient comfort, but also in mitigating the adverse effects of pain on respiratory function and airway clearance. Inadequate pain relief can lead to splinting and patient reluctance to cough and deep breathe; the end result is promotion of retained secretions, atelectasis, hypoxemia, and, possibly, pneumonia. For major surgical procedures, particularly those involving the chest and upper abdomen, administration of opiates via the parenteral or epidural route has become the analgesic method of choice.
The use of narcotic analgesia in the postoperative period is associated with a small, but not insignificant, risk of precipitating respiratory depression. The reported incidence of respiratory depression varies based on the criteria employed. A meta-analysis of published studies revealed an incidence of 0.3% defined by the need to administer naloxone, 3.3% defined by the presence of hypercapnia, and 17% defined by oxygen desaturation.26 The risk may be slightly lower in association with the epidural as opposed to parenteral route of administration. When administered epidurally, hydrophilic narcotics (e.g., morphine) have a greater tendency than lipophilic compounds (e.g., fentanyl) to remain in the cerebrospinal fluid and to spread rostrally to the respiratory center located in the floor of the fourth ventricle. Elderly patients are particularly susceptible to the respiratory depressant effects of opiates, likely reflecting an impaired ability to metabolize these agents. Respiratory depression in the postoperative patient is most likely to occur during the initial 24 hours following surgery. It is typically accompanied by a decreased level of consciousness and a slow respiratory rate. Treatment consists of administration of naloxone in 0.1 to 0.4 mg aliquots. Ventilation should be supported with a face mask and Ambu bag, reserving intubation for the failure of naloxone to swiftly rectify the problem.
Prospective, randomized trials examining pulmonary complications associated with epidural versus intravenous administration of perioperative analgesia following major abdominal surgical procedures have provided somewhat conflicting results. One study of 168 patients found no difference in rates of prolonged intubation or reintubation between groups receiving epidural versus intravenous postoperative analgesia.27 A small trial involving 70 elderly patients reported no difference in duration of postoperative mechanical ventilation or major pulmonary complications (including reintubation).28 In contrast, a larger study of 915 patients with at least one comorbidity defining them as “high-risk” surgical candidates found that epidural analgesia was associated with a significant reduction (23% with epidural vs. 30% with intravenous route) in postoperative respiratory failure, defined rather broadly as need for prolonged ventilation or reintubation or PaO2 ≤50 mm Hg or PaCO2 ≥50 mm Hg on room air.29 Finally, a trial of over 1000 patients demonstrated a significant reduction in postoperative respiratory failure (defined as the need for intubation and mechanical ventilation for >24 hours postoperatively or reintubation) with the use of epidural analgesia in the subset of patients undergoing aortic surgery, but not those undergoing nonaortic abdominal surgery.30
IMPACT OF SURGERY ON POSTOPERATIVE PULMONARY FUNCTION
Surgery involving the upper abdomen and thorax results in a pronounced impairment in pulmonary function in the postoperative period. The impairment is more severe and prolonged than that due to administration of general anesthesia alone. Typically, upper abdominal and thoracic procedures are associated with a fall in lung volumes, development of atelectasis, and hypoxemia. These adverse effects commonly necessitate short-term administration of low-flow, supplemental oxygen, but when severe, or when accompanied by underlying lung disease, may precipitate respiratory failure.
 UPPER ABDOMINAL SURGERY
UPPER ABDOMINAL SURGERY
Within 24 hours following upper abdominal surgery, vital capacity declines by 50%.31 Although the vital capacity improves with time, marked impairment persists for as long as 7 days after the surgery. In contrast, vital capacity falls by only 25% following lower abdominal procedures; it returns to normal by the third postoperative day.31 Underlying these profound changes after upper abdominal surgery is the development of diaphragmatic dysfunction, as reflected in a reduction in transdiaphragmatic pressure with tidal respirations and in a shift from abdominal to rib cage breathing.32,33
Two main theories have been proposed to explain the observed impairment in diaphragmatic function. One theory is that there is a primary alteration in diaphragmatic contractility induced by local irritation, inflammation, surgical trauma, or pain. This theory has been rendered improbable with the demonstration that external stimulation of the phrenic nerves produces normal peak transdiaphragmatic pressure in patients recovering from upper abdominal surgery.32 In other words, when maximally stimulated the diaphragm functions in a normal fashion.
The alternative, and currently favored, theory proposes that diaphragmatic dysfunction results from diminished phrenic nerve output. The basis for the attenuation in neural drive remains a matter of speculation, although several putative pathways can be rationally eliminated. For example, general anesthesia is known to depress output from the central respiratory centers, as well as to inhibit synaptic transmission. However, as noted previously, the effects of general anesthesia on diaphragmatic tone are transient and modest. In addition, the degree of dysfunction observed after upper abdominal procedures is not seen following general anesthesia for procedures on the lower abdomen and extremities. An inhibitory arc initiated by abdominal nociceptors for pain is unlikely, given that achievement of adequate pain control by epidural opiates fails to consistently improve pulmonary function or to normalize diaphragmatic performance. In contrast, the epidural administration of anesthetic agents such as bupivacaine does ameliorate diaphragmatic dysfunction following upper abdominal surgery. Since these agents produce sympathetic blockade in addition to pain control, it has been argued that visceral sympathetic afferents are responsible for providing an inhibitory signal that downgrades central neural drive and phrenic nerve activity, thereby leading to impaired diaphragmatic function. Supporting the notion of a reflex inhibitory arc mediated by visceral afferents is the demonstration in experimental animals that mechanical gall bladder stimulation strongly inhibits electromyographic activity and motion of the diaphragm.34
 CARDIAC SURGERY
CARDIAC SURGERY
Although CABG – the most commonly performed cardiac surgical procedure – has been most intensively scrutinized with respect to its impact on the respiratory system, other related cardiac procedures (e.g., valve replacement) are likely to have similar effects. Lung volumes decrease by approximately 30% after CABG; the return to preoperative values may take several months.35–37 Lung function may decline to a greater degree when internal mammary harvesting and grafting are employed.35 Gas exchange is also impaired after CABG, as evident in the development of hypoxemia and significant widening of the alveolar–arterial oxygen gradient. In 125 patients who had daily room air arterial blood gas determinations prior to and following CABG, PaO2 fell from approximately 75 mm Hg preoperatively to a nadir of 55 mm Hg on postoperative day 2.38 The PaO2 improved but remained below preoperative values at the end of the first postoperative week. A similar pattern and magnitude of decline in oxygenation have been demonstrated in other studies, with the development of hypoxemia associated with an increase in calculated shunt fraction from 3% preoperatively to a peak of 19% postoperatively.37 The increase in shunt fraction is readily accounted for on the basis of atelectasis, which is invariably present postoperatively, especially on the left side.
A number of factors have been implicated in the development of post-CABG pulmonary dysfunction and atelectasis. Alterations in chest wall compliance and motion may result from division of the sternum, harvesting of the internal mammary artery, and traumatic injury to the costovertebral joints and first rib induced by retraction. Intraoperative lung retraction may directly injure the left lower lobe, leading to contusion and atelectasis, and, perhaps, accounting for the predilection for radiographic infiltrates on the left side. An alternative explanation for post-CABG left lower lobe atelectasis is intraoperative injury to the left phrenic nerve and consequent diaphragmatic paralysis or paresis. The phrenic nerve is vulnerable to stretch and ischemic injury during sternal retraction, dissection of the left internal mammary artery, or prolonged distention of the pericardium. In addition, thermal injury to the nerve may occur with the cardioplegic technique of instilling iced slush into the open pericardial sac. The actual incidence of phrenic nerve dysfunction after CABG is best defined in studies employing electrophysiological techniques, which have documented evidence of phrenic nerve injury in 10% to 16% of patients.39–41 This suggests that phrenic nerve injury accounts for only a minority of the observed cases of left lower lobe atelectasis.
Finally, cardiopulmonary bypass (CPB) may contribute to pulmonary impairment after cardiac surgery. The duration of CPB has been linked to the severity of postoperative atelectasis; whether this relationship is causal is unclear.40 It has been hypothesized that the use of CPB leads to abnormal surfactant production – possibly due to ischemic, thermal, or toxic injury to the alveolar epithelium – predisposing to the development of atelectasis. More clearly established is the ability of the bypass pump to induce a capillary leak syndrome, marked by extravasation of fluid into the alveolar interstitium and, rarely, into the airspaces. This process is thought to result from exposure of blood to nonendothelial surfaces, resultant activation of neutrophils, complement, and other inflammatory cascades, and sequestration of neutrophils within the microvasculature. While this rarely may lead to full-blown ARDS (see discussion below), the consequences are usually more subtle, manifesting as a widened arterial–alveolar oxygen gradient and diminished lung compliance. The recent introduction of “off-pump” CABG has permitted a greater appreciation of the adverse impact of CPB on postoperative lung function. For example, a recent large multicenter comparative analysis from the United Kingdom of CABG with or without CPB demonstrated significant reductions in the rates of prolonged mechanical ventilation (>24 hours), reintubation/tracheostomy, and ARDS/pulmonary edema/pneumonia among the group that underwent off-pump CABG.42
 LUNG RESECTION
LUNG RESECTION
Unique to lung resection surgery is the immediate loss of lung function due to removal of lung parenchyma. The magnitude of the loss can be estimated reliably from preoperative quantitative lung scanning in conjunction with standard spirometry. The impact of lung resection on pulmonary function is further magnified in the perioperative period by other factors. For example, the standard posterolateral thoracotomy incision represents significant chest wall trauma, with rib retraction and resection, and transection of intercostal, latissimus dorsi, trapezius, and serratus anterior muscles. As a result, total respiratory compliance may fall by as much as 75%; work of breathing increases; and lung volumes decline dramatically, out of proportion to the surgical loss of functional lung. Following standard thoracotomy and lung resection (either lobectomy or wedge resection), FEV1 and FVC fall to 25% of preoperative values at 1 hour, and to 30% at 24 hours. When a more limited, muscle-sparing incision is used, the impact on pulmonary function is markedly attenuated.43 Lung resection via VATS is also associated with less severe impairment in lung function.17
As with cardiac and upper abdominal surgery, atelectasis is frequently present after lung surgery and results in impaired oxygenation. Phrenic nerve activity remains normal and diaphragmatic function during tidal breathing is preserved, although maximal diaphragmatic strength may be reduced.
CAUSES OF POSTOPERATIVE RESPIRATORY FAILURE
The development of acute respiratory failure in the surgical patient should prompt a systematic assessment of the likely causes (Table 104-4). In approaching this life-threatening problem, one must consider the nature and magnitude of pre-existing pulmonary disease, type of surgery performed, drugs administered intra- and postoperatively, and predominant derangement in gas exchange (i.e., hypoxemia or hypercapnia). In conjunction with important information derived from the physical examination and chest radiograph, the analysis should readily identify factors responsible for or contributing to respiratory failure. The following discussion focuses on the more common or unique causes of postoperative respiratory failure in the surgical setting.
 ATELECTASIS
ATELECTASIS
Atelectasis is the most common pulmonary complication encountered in the surgical patient, particularly following thoracic and upper abdominal procedures. As discussed previously, anesthesia and surgical manipulation act in concert to produce regional atelectasis through incompletely defined mechanisms, including diaphragmatic dysfunction and diminished surfactant activity. The atelectasis is typically basilar and segmental in distribution, obscuring the hemidiaphragms radiographically. A distinct and less common cause of postoperative atelectasis is plugging of central airways by retained secretions. This problem is encountered in the surgical patient whose efforts to clear secretions are compromised by depressed consciousness, inadequate pain control, or a weak, ineffective cough. When situated in a main stem bronchus, mucus plugs can result in collapse of an entire lung; more distal obstruction leads to lobar collapse. An abrupt termination of the proximal bronchial air shadow and the absence of air bronchograms within the atelectatic portion of lung are clues to the possible presence of mucus plugging.
While often clinically insignificant, postoperative atelectasis may lead to severe hypoxemia and respiratory distress. The magnitude of hypoxemia is dictated by the extent of atelectasis, the presence and severity of underlying lung disease, and the integrity of the hypoxemic pulmonary vasoconstrictive response. Impairment of hypoxemic pulmonary vasoconstriction by vasodilatory drugs, commonly administered to surgical patients for treatment of underlying hypertension or ischemic heart disease, prevents the compensatory diversion of blood flow away from nonventilated areas of lung and magnifies the shunt fraction.
Respiratory distress due to atelectasis usually evolves insidiously over the first several postoperative days. Supplemental oxygen requirements increase in association with worsening basilar infiltrates noted on the chest radiograph. The clinicoradiographic picture may be indistinguishable from that of pneumonia. While fever and leukocytosis suggest infection, these signs are common and nonspecific. When atelectasis is due to central airway occlusion by mucus plugs, hypoxemia and respiratory distress may develop quickly. A chest radiograph obtained immediately after the onset of symptoms may be surprisingly unrevealing if sufficient time has not passed to permit resorption of gas from the airspaces of the nonventilated lung. Careful examination of the patient, however, will reveal an absence of breath sounds over the involved lung, providing an important clue to the presence of central airway obstruction and obviating pursuit of other considerations, such as pulmonary embolism (PE).
Treatment of respiratory failure due to atelectasis is directed toward the combined goals of adequate oxygenation and re-expansion of lung segments. Supplemental oxygen should be titrated to achieve an arterial oxyhemoglobin saturation of at least 90%. Refractory hypoxemia, severe respiratory distress, progressive hypercapnia, or inability of the patient to clear copious airway secretions should prompt immediate intubation and mechanical ventilatory support. This lifesaving intervention permits more efficient delivery of oxygen, secures access for suctioning of the airways, and facilitates performance of bronchoscopy should it be necessary. Moreover, the positive pressure and large tidal volumes delivered by the ventilator are often effective in rapidly re-expanding collapsed lung segments. In less dire circumstances, noninvasive ventilation may be equally effective.
Fiberoptic bronchoscopy is commonly employed in the treatment of serious atelectasis but evidence suggests that it may be no more effective than standard chest physiotherapy. In the only randomized study directly comparing these strategies, immediate fiberoptic bronchoscopy did not result in more rapid or complete resolution of acute lobar atelectasis when compared with a regimen of deep breathing, coughing, suctioning of the intubated patient, aerosolized bronchodilator treatments, chest percussion, and postural drainage.44 Resolution of atelectasis was dictated not by the treatment modality employed, but by radiographic evidence of central airway patency. In this regard, both chest physiotherapy and bronchoscopy were highly effective in the absence of an air bronchogram. In contrast, the presence of an air bronchogram, which indicates that the atelectasis is not due to proximal airway obstruction, was associated with minimal response to either modality. Based on this, standard respiratory therapy techniques applied to either the spontaneously or mechanically ventilated patient should be considered the mainstay of treatment for lobar atelectasis. Fiberoptic bronchoscopy should be reserved for those situations where chest physiotherapy is contraindicated (e.g., chest trauma, immobilized patient), poorly tolerated, or unsuccessful, or in the setting of life-threatening hypoxemia.
A number of other measures are commonly employed in the treatment of atelectasis. Judicious use of analgesia is an essential adjunct, permitting the patient to breathe deeply, cough forcefully, and comfortably participate in chest physiotherapy maneuvers. Care must be taken to avoid excessive sedation, which will offset the beneficial effects of pain control. In the setting of marked hypoxemia, attempts should be made to discontinue vasoactive drugs with the potential to influence the pulmonary vascular bed; examples include nitrates, nitroprusside, calcium channel blockers, angiotensin-converting enzyme inhibitors, and hydralazine. Mucolytics, such as N-acetyl cysteine, are commonly administered in an effort to promote clearance of tenacious secretions; however, their efficacy in this setting has not been well documented. Some clinicians and respiratory therapists advocate the use of nasotracheal suctioning of the nonintubated patient with a weak and ineffective cough. However, this technique is associated with considerable discomfort and is an inefficient and highly transient means of clearing secretions from the tracheobronchial tree.
Maneuvers to promote periodic full lung expansion, including intermittent positive pressure breathing (IPPB), cough and deep breathing exercises, and incentive spirometry, have been developed in an attempt to prevent or mitigate the severity of atelectasis. Early trials suggested that all three techniques were equally efficacious and superior to no therapy in the prevention of postoperative pulmonary complications following abdominal surgery.45 IPPB has largely been abandoned due to its expense, need for specially trained personnel and close patient supervision, and tendency to produce abdominal distention. While incentive spirometry is universally employed in the care of the postoperative patient, two recent Cochrane Reviews challenge the deeply ingrained view that this is an essential measure. These reviews, focusing on upper abdominal surgery46 and CABG,47 respectively, conclude that there is no evidence of benefit from incentive spirometry in reducing pulmonary complications and, in the case of CABG, in decreasing the negative effects on pulmonary function. Both reviews caution that there are extensive methodological shortcomings of available studies that limit their interpretation. Given that incentive spirometry is inexpensive and simple to perform, it seems prudent to continue this practice despite the absence of rigorous proof of efficacy.
 PNEUMONIA
PNEUMONIA
Pneumonia is the third most common postoperative infection and the most lethal, with an associated mortality rate of 20% to 50%.48 Pneumonia represents a principal cause of postoperative respiratory compromise and may precipitate acute respiratory failure, as well as complicate respiratory failure in the patient who is ventilator-dependent for other reasons. The type of surgery heavily influences the risk of developing pneumonia in the postoperative period. In particular, the risk is greatest following abdominal aortic aneurysm repair, thoracic and upper abdominal procedures, head and neck surgery, and neurosurgical procedures.48 Patient-specific risk factors that have been identified include advanced age, chronic steroid use, current or recent smoking, COPD, low serum albumin level, impaired sensorium, and dependent preoperative functional status.48,49 The presence of a nasogastric tube and the use of gastric acid–suppressive medications, commonly employed interventions in postoperative patients, have been cited as risk factors for nosocomial pneumonia in general.50 A multifactorial model, employing a number of these patient-related and procedure-related factors, has been developed and validated that predicts the risk of pneumonia in nonintubated patients following major noncardiac surgery.48 Perhaps the most important risk factor, not addressed in this predictive model, is the need for postoperative mechanical ventilatory support. Overall, mechanically ventilated patients have a 6- to 21-fold increased risk of pneumonia compared with nonventilated patients.51
While organisms may reach the lower respiratory tract by several routes, microaspiration of oropharyngeal secretions appears to be the predominant mechanism in the pathogenesis of nosocomial pneumonia. A critical initiating event in this pathway is colonization of the oropharynx with gram-negative aerobic bacilli, a process that characteristically occurs in response to serious illness or surgical stress. Clinically occult aspiration of these virulent organisms is facilitated by a number of iatrogenic measures imposed upon the surgical patient. Paramount among these is the placement of an endotracheal tube, which impairs swallowing, stents open the glottis, and permits pooling of secretions above the tube cuff. The inflated cuff is an imperfect barrier and allows intermittent seepage of secretions into the lower airways. Prolonged intubation has also been associated with postextubation swallowing dysfunction. Depressed consciousness as a consequence of general anesthesia and postoperative analgesia further contributes to the risk of aspiration.
Recent attention has focused on the stomach as an additional source of bacteria in the development of nosocomial pneumonia. While the acidic milieu of the stomach normally inhibits bacterial growth, the common use of H2-blockers and antacids as stress ulcer prophylaxis overrides this natural barrier and promotes gastric colonization with gram-negative enteric organisms. Gastroesophageal reflux, a common feature of the critically ill patient, permits bacteria-laden gastric contents to enter the respiratory tract either directly or by first colonizing the oropharynx. This route of migration has been confirmed by recovery of technetium-99m-labeled gastric contents in endobronchial secretions and by the demonstration in some patients that organisms cultured from the airways first appeared in the stomach.52 Perhaps the most compelling, albeit circumstantial, evidence derives from studies that have shown a higher incidence of nosocomial pneumonia in patients receiving H2-blockers or proton-pump inhibitors.50
The fate of organisms introduced into the lower respiratory tract is dependent upon the integrity of mechanical and immunological pulmonary defense mechanisms. Impairment of the mucociliary escalator (e.g., due to recent cigarette smoking or underlying COPD), weak and ineffective cough, and use of immunosuppressive medications (e.g., corticosteroids) favor the proliferation of organisms and the development of pneumonia. It is widely held that postoperative atelectasis predisposes to pneumonia by entrapping bacteria. However, studies demonstrating a lack of concordance between the degree of atelectasis and the subsequent risk of pneumonia challenge this contention.53
The constellation of fever, leukocytosis, purulent sputum, and radiographic infiltrates has traditionally defined the presence of pneumonia. While these diagnostic criteria are reasonably accurate in the previously healthy outpatient, they are notoriously nonspecific in the setting of recent surgery, particularly with prolonged use of mechanical ventilation. In one autopsy series, traditional clinical and radiographic criteria provided the correct antemortem diagnosis in only 70% of cases.54 Alternative etiologies of radiographic infiltrates include atelectasis, pulmonary edema, infarction or hemorrhage due to pulmonary emboli, pulmonary contusion, and chemical pneumonitis. Cultures of sputum and tracheal aspirates are poorly reflective of the bacterial flora of the distal airways, since these specimens are contaminated by colonizing organisms in the oropharynx and upper respiratory tract. In an attempt to enhance diagnostic certainty, bronchoscopic sampling of the distal airways using a sterile sheathed brush or bronchoalveolar lavage has been advocated. While the absence of a “gold standard” for the diagnosis of pneumonia has complicated attempts to define the accuracy of these techniques, rates of false-positive and false-negative results have generally fallen in the range of 30%. It is questionable, therefore, whether the performance of bronchoscopy actually contributes significantly to a reduction in the degree of diagnostic uncertainty.
The most common organisms isolated in cases of postoperative pneumonia are gram-negative bacteria and Staphylococcus aureus.55 Polymicrobial infections are common, occurring in approximately one-third of cases. The emergence of highly resistant organisms, such as methicillin-resistant S. aureus and multidrug resistant Acinetobacter species poses a particular challenge in selecting empiric antibiotics and in definitively treating these infections. In one large study of postoperative pneumonia, the initial antibiotic regimen had to be modified in 47% of cases because of antibiotic resistance or clinical failure.55
Preventative strategies intended to diminish the risk of pneumonia are an important consideration in the care of the surgical patient. Prevention begins in the preoperative phase with emphasis on abstinence from cigarette smoking for a minimum of 8 weeks prior to elective surgery. Following surgery, nasogastric and endotracheal tubes should be removed as soon as possible. Postoperative analgesia must be titrated to permit the patient to comfortably and vigorously cough, but excessive sedation impairing protection of the airway and enhancing the risk of aspiration must be avoided. For the high-risk ventilator-dependent patient, maintenance of a semierect position has been shown to diminish the magnitude of clinically occult aspiration of gastric contents and the incidence of pneumonia. More controversial in this high-risk population is the use of selective digestive decontamination (SDD), intended to prevent or diminish the magnitude of gram-negative colonization of the aerodigestive tract. Regimens have varied among studies, but they typically consist of some combination of antibiotics applied topically to the oropharynx, instilled into the stomach as a slurry, and/or administered systemically. Several prospective randomized trials and meta-analyses demonstrated that SDD reduced the incidence of pneumonia among critically ill patients.56,57 Despite the suggested efficacy of this approach, SDD is not widely utilized, in part because of lingering concerns that this strategy will lead to emergence of increasingly resistant bacterial strains.
 ACUTE RESPIRATORY DISTRESS SYNDROME
ACUTE RESPIRATORY DISTRESS SYNDROME
ARDS is defined by the constellation of hypoxemic respiratory failure, diffuse pulmonary infiltrates, and the absence of clinical evidence of elevated left atrial pressure (see Chapter 141). The histological hallmark of ARDS is diffuse alveolar damage, a widespread injury to the alveolar–capillary membrane that leads to increased capillary permeability and development of noncardiogenic pulmonary edema. ARDS represents the end result of a variety of insults that either involve the lung directly (e.g., aspiration of gastric contents) or trigger pulmonary inflammation as part of a systemic process (e.g., sepsis). Many of the risk factors associated with development of ARDS are commonly encountered in surgical patients. In decreasing order of risk, these include sepsis, massive blood transfusion, pulmonary contusion, aspiration of gastric contents, and multiple fractures.58 Causes of ARDS of particular relevance to the surgical patient, and, in some cases, unique to this population, are described in greater detail below.
 ASPIRATION OF GASTRIC CONTENTS
ASPIRATION OF GASTRIC CONTENTS
Aspiration of gastric contents can rapidly lead to widespread acute lung injury and is an important cause of ARDS in the surgical patient. It is the third leading cause of anesthesia-related deaths, accounting for 10% to 30% of fatal outcomes. Aspiration typically occurs when the mechanisms of glottic closure and cough, which normally protect the airway, are compromised. In the surgical patient, the period of maximal vulnerability for aspiration spans from the induction of general anesthesia to full return of consciousness postoperatively. A number of factors combine to enhance the risk of aspiration during this period. Most important is the blunting of consciousness that accompanies induction and administration of general anesthesia. Insufflation of air into the stomach during induction may cause gastric distention and promote vomiting. Vomiting may also be provoked by noxious stimulation of the posterior oropharynx during intubation or extubation. Reflux of gastric contents is facilitated by medication-induced relaxation of the lower esophageal sphincter, placement of the patient in a supine position, and manipulation of the bowel during abdominal procedures. At the completion of surgery, extubation is commonly performed at a time when the patient, while able to ventilate adequately, may not yet be capable of fully protecting the airway. Indeed, upper airway reflexes remain significantly impaired for up to 2 hours after recovery from anesthesia, even at a time when mental alertness has returned. Moreover, translaryngeal intubation, even when brief, may cause residual glottic dysfunction for up to 8 hours following removal of the tube. While the risk of aspiration diminishes beyond the immediate perioperative period, it remains a concern in the patient receiving narcotic analgesia, which may not only induce vomiting, but also depress consciousness.
The risk of aspiration during the immediate perioperative period was delineated in a survey of over 215,000 general anesthetic procedures performed at the Mayo Clinic.59 Aspiration was defined as the presence of bilious or particulate matter in the airways or the development of a new infiltrate on the immediate postoperative chest radiograph. The overall incidence of aspiration was only 0.03%, but the incidence was nearly fourfold higher (0.11%) in the setting of emergency surgery. In addition to the use of general anesthesia, other predisposing factors were present in over half of the patients who aspirated. These included gastrointestinal obstruction, swallowing dysfunction, altered sensorium, previous esophageal surgery, and a recent meal. The majority of events occurred during laryngoscopy (in preparation for insertion of the endotracheal tube) and during tracheal extubation. Twenty percent of patients who aspirated required postoperative mechanical ventilation in excess of 6 hours; 5% died as a direct result of this complication. The use of laryngeal mask airway devices does not appear to be associated with an increased risk of aspiration despite the fact that these devices do not isolate the larynx from the gastrointestinal tract.60
Acidic gastric contents introduced into the airways are rapidly disseminated throughout the bronchial tree and lung parenchyma, producing an almost instantaneous chemical burn. In addition, acid aspiration triggers a more delayed inflammatory response, with release of inflammatory cytokines and recruitment of neutrophils into the lung. The result is injury to the alveolar–capillary membrane, with flooding of the interstitium and airspaces by proteinaceous edema fluid. Surfactant levels drop precipitously due to both direct acid denaturation and diminished production, leading to alveolar instability and atelectasis. The magnitude of lung injury is directly related to the pH and volume of aspirated material. Initial studies in animals suggested that a pH of less than 2.5 and a volume in excess of 0.4 mL/kg are critical threshold values for the induction of lung injury.61 While these values are now often quoted in the literature, their validity has been challenged by more recent studies demonstrating significant injury in association with lower volumes and higher pH. In particular, aspiration of bile is capable of inducing widespread injury even at a pH as high as 7.19. The presence of large food particles may further exacerbate the problem by causing airway obstruction and atelectasis. Notably, infection does not normally play a significant role in the initial lung injury from aspiration of acidic gastric contents, as the low pH serves to maintain relative sterility of the inoculum. However, gastric colonization with bacteria can occur in patients maintained on acid-suppressive agents, those receiving enteral feeds, and those with gastroparesis or small bowel obstruction.
The diagnosis of aspiration is most firmly established in the setting of witnessed vomiting or recovery of gastric contents from the airways. More often, the diagnosis is suspected circumstantially in a patient with risk factors and a compatible clinicoradiographic picture. Massive aspiration presents with fever, tachypnea, and diffuse rales developing within several hours of the event. Wheezing is appreciated in approximately one-third of patients and may be due either to obstruction of airways by particulate matter or, more commonly, to reflex bronchospasm. Hypoxemia is universally present with massive aspiration and is sufficiently severe in the majority of patients to mandate use of mechanical ventilation. The initial presence of apnea or shock is particularly ominous and portends a high risk of subsequent death. Initial radiographic patterns vary, depending upon the volume, causticity, and distribution of the aspirated material. However, three general patterns have been described: (1) extensive bilateral consolidation resembling diffuse pulmonary edema; (2) widespread, but discrete, patchy infiltrates involving dependent areas of lung; and (3) focal consolidation usually localized to one or both lung bases.
The clinical course following massive aspiration is variable, but it typically diverges along one of several pathways. A minority of patients follow a fulminant course leading quickly to hypoxemic respiratory failure. More commonly, patients demonstrate progressive radiographic and clinical improvement over the first several days. Although most of these patients will go on to full recovery, a subset demonstrates secondary deterioration due to the development of nosocomial pneumonia. The overall mortality rate associated with massive aspiration is approximately 30% and exceeds 50% in those patients with initial shock or apnea, secondary pneumonia, or ARDS.
The treatment of respiratory failure secondary to aspiration is supportive and includes mechanical ventilatory strategies common to other forms of ARDS (Chapter 141). Bronchoscopy is indicated only when large airway obstruction by particulate matter is suspected on the basis of a localized wheeze or lobar atelectasis. Because acid is disseminated and endogenously neutralized within seconds, large-volume bronchoalveolar lavage is ineffective in attenuating the degree of injury and is not recommended. Studies of the administration of systemic corticosteroids in the treatment of aspiration pneumonitis have been inconclusive and do not currently justify their use. Similarly, use of prophylactic antibiotics is generally discouraged in the absence of supportive data and because of fear that this practice will preferentially select more highly resistant organisms. Some authors do advocate use of empiric antibiotics for that subset of patients at risk for gastric colonization with bacteria, as described above. In addition, up to 40% of patients will develop a superimposed bacterial pneumonia within several days of the aspiration event, often heralded by a new fever, new or progressive infiltrates, and purulent sputum. Broad-spectrum antibiotic therapy is indicated at that time.
The high morbidity and mortality associated with aspiration and the lack of effective therapy once the event has occurred have focused attention on measures to prevent this complication. The most straightforward and widely utilized measure is the convention of overnight fasting prior to elective surgery. However, despite prolonged fasting, up to one-third of patients will maintain a gastric volume in excess of 0.4 mL/kg (approximately 25–30 mL in the average adult), and up to three-quarters will have a gastric pH below 2.5. Administration of H2-blockers and proton-pump inhibitors can effectively raise the pH and reduce the volume of gastric contents, suggesting a potentially appealing strategy.60 However, evidence suggesting that preoperative administration of these agents decreases the incidence or severity of aspiration is notably absent and their routine use in the preoperative setting is not currently recommended. In high-risk patients, rapid sequence induction of anesthesia should be employed to shorten the time between loss of consciousness and tracheal intubation. During induction, manual pressure should be applied to the cricoid cartilage (Sellick maneuver) and maintained until the endotracheal tube is in proper position and the cuff is inflated. Postoperatively, extubation should be performed only when consciousness and the gag reflex have returned to a level sufficient to permit adequate protection of the airway.
 POSTPNEUMONECTOMY PULMONARY EDEMA
POSTPNEUMONECTOMY PULMONARY EDEMA
Postpneumonectomy pulmonary edema describes the development of diffuse pulmonary edema in the remaining lung, in the absence of identifiable causes. While some authors apply this term exclusively in the setting of pneumonectomy, others have reported similar cases following lobectomy. Demonstration of normal pulmonary artery occlusion pressures and of protein-rich edema fluid in a small number of patients suggests that an increase in vascular permeability rather than hydrostatic pressure underlies the edema formation.62 However, the exact mechanism responsible for lung injury remains a matter of conjecture. Mechanisms that have been advanced include hyperinflation and volutrauma resulting from single lung ventilation, endothelial injury due to hyperperfusion of the remaining lung, disruption of lymphatic drainage, and ischemia-reperfusion injury.63
The incidence of postpneumonectomy pulmonary edema ranges between 4% and 7%, in part reflecting variable definitions.63 For unclear reasons, the complication is encountered more frequently following right pneumonectomy. The majority of cases develop by postoperative day 3. The observed mortality rate is in the range of 50% to 100%. Treatment is supportive, centering on lung-protective ventilator strategies. A nonrandomized trial suggested that the intraoperative administration of Solu-Medrol was associated with a decreased incidence of postpneumonectomy pulmonary edema but this is not considered standard of care.64
 CARDIOPULMONARY BYPASS
CARDIOPULMONARY BYPASS
ARDS develops immediately following use of CPB in approximately 1% of cases.65 While factors unrelated to the use of CPB may be at play, there is evidence from both animal models and clinical studies to suggest that CPB activates a number of inflammatory pathways that could lead to acute lung injury. It is well established, for example, that CPB results in neutrophil activation, likely through mechanical shear stress and exposure to the artificial surfaces of the bypass circuit. In addition, an increased expression of cell surface adhesion molecules has been demonstrated, which may promote neutrophil binding to pulmonary endothelium and release of proteolytic enzymes and reactive oxygen species. The central role played by neutrophils in causing acute lung injury following CPB is supported by several lines of evidence: (1) bronchoalveolar lavage fluid from patients undergoing CPB contains an increased number of neutrophils; (2) plasma levels of neutrophil elastase and myeloperoxidase are increased; and (3) inhibition of neutrophil activation with pentoxiphylline as well as neutrophil depletion attenuate the degree of pulmonary dysfunction. A number of other inflammatory mediators are released in association with CPB, including complement, proinflammatory cytokines, and prostaglandins. Perhaps the most compelling, albeit circumstantial, evidence implicating CPB as a cause of postoperative pulmonary dysfunction is the demonstration in multiple studies of a significant reduction in the incidence of prolonged postoperative ventilator dependence associated with CABG when performed off-pump.42,66,67
 AMIODARONE
AMIODARONE
Amiodarone-induced pulmonary toxicity usually presents as a subacute illness characterized by cough, dyspnea, fever, and patchy pulmonary infiltrates. Less commonly, the use of amiodarone has been linked to the development of ARDS immediately following cardiac and thoracic surgery. In most of the reported cases, amiodarone was administered preoperatively for varying periods of time for control of arrhythmias.68 The majority of patients had no evidence prior to surgery of the more indolent form of amiodarone pulmonary toxicity. Development of ARDS also has been described in patients whose only exposure to amiodarone occurred in the postoperative period, when the drug was initiated as prophylaxis or treatment for atrial arrhythmias. In one report, postoperative ARDS developed in 11% of patients receiving amiodarone and in only 1.8% of untreated patients.69 The specific perioperative factors that act in concert with amiodarone to produce acute lung injury remain to be defined. Some authors have suggested that exposure to high levels of supplemental oxygen may be a contributing factor. The diagnosis rests on exclusion of other causes rather than on specific diagnostic tests or histology. In addition to stopping the drug, high doses of corticosteroids are often given, though support for this is anecdotal at best.
 TRANSFUSION-RELATED ACUTE LUNG INJURY
TRANSFUSION-RELATED ACUTE LUNG INJURY
The transfusion of blood and blood products has been linked to the development of acute lung injury in two ways. An association between massive blood transfusion (>15 units/24 hours) and ARDS has been noted in epidemiological studies, but it remains unclear whether this link is truly causal or is indirect and reflective only of the critically ill nature of the patient requiring such massive transfusion support.58 Acute lung injury can also be associated with the transfusion of a single unit of blood or blood components, an entity known as “transfusion-related acute lung injury” (TRALI). TRALI is operationally defined as the acute development of hypoxemia and bilateral radiographic opacities during or within 6 hours of a completed transfusion, in the absence of evidence of circulatory overload or alternative etiologies of acute lung injury.70
The mechanism underlying TRALI was initially determined to involve passive infusion via transfused blood products of donor-derived antibodies directed against recipient leukocytes. These antibodies are typically contained in blood products obtained from multiparous female donors, whose exposure to foreign human leukocyte antigen (HLA) or neutrophil antigens occurred during prior pregnancies. When transfused into a recipient with cognate antigens, these antibodies result in leukoagglutination and activation of recipient granulocytes or monocytes within the pulmonary microvasculature, triggering increased capillary permeability and the development of noncardiogenic pulmonary edema. Less commonly, the leukoagglutinating process can be triggered by an interaction between recipient anti-HLA or antineutrophil antibodies and donor-derived leukocytes contained in the transfused blood products.71
While ample clinical and animal model evidence exists to support the antibody hypothesis,70,72 several lines of evidence suggest that other factors may also be involved. First, not all patients who receive blood products containing recipient-specific anti-HLA or antineutrophil antibodies develop TRALI. Second, in a significant minority of cases, leukoagglutinating antibodies cannot be detected in either the donor or recipient. This has prompted the proposal of an alternative “two hit” mechanism, whereby an initial event is required to prime recipient neutrophils and a second event leads to activation of primed neutrophils.70,72 Priming events that have been associated with an increased risk of TRALI include recent surgery, active infection, sepsis, and chronic alcohol abuse.73,74 The second event involves transfusion of substances that trigger neutrophil activation: either antileukocyte antibodies or biologically active lipids resulting from breakdown of stored blood products.
The true incidence of TRALI is difficult to determine due to underrecognition of the entity and a tendency to attribute the findings to other causes of acute lung injury. Although virtually all blood products have been implicated, the risk of TRALI is highest in association with fresh-frozen plasma and platelets.71,72 Many cases are detected in surgical patients in the immediate postoperative period, a fact that likely reflects the frequent need for transfusions in this setting and the close monitoring of cardiopulmonary function in the postanesthesia recovery area.75,76
Clinically, TRALI is characterized by the abrupt onset of dyspnea and hypoxemia during or shortly following transfusion of blood products. Accompanying features include fever, chills, and hypotension. Respiratory distress and hypoxemia are of sufficient magnitude to require mechanical ventilatory support in up to 45% of cases.71 The differential diagnosis includes volume overload, congestive heart failure, myocardial infarction, and aspiration. Although many cases are self-limited, with clearing of infiltrates and improved oxygenation over several days, mortality rates of 5% to 20% have been reported.71
When TRALI is suspected, the blood bank should be notified. Ideally, this should trigger testing of all donor specimens (typically retained by the blood bank) for the presence of anti-HLA and antineutrophil antibodies. However, the expense and time required often prevent blood banks from performing these studies.
 ACUTE EXACERBATIONS OF PULMONARY FIBROSIS
ACUTE EXACERBATIONS OF PULMONARY FIBROSIS
Patients with idiopathic pulmonary fibrosis (IPF) can experience sudden and dramatic respiratory decompensation in the absence of identifiable precipitants. Referred to as an exacerbation of IPF, this phenomenon is characterized by acutely worsening hypoxemia in association with new bilateral ground-glass opacities or consolidation on chest CT.77 The histological hallmark is diffuse alveolar damage, superimposed on underlying usual interstitial pneumonia. IPF exacerbation is a diagnosis of exclusion, established only after infection, heart failure, PE, and identifiable causes of acute lung injury have been ruled out.
The immediate postoperative period following lung surgery is one of the settings in which acute exacerbations of IPF have been reported to occur.78–80 Most cases have occurred following either lung resection for lung cancer or lung biopsy for diagnosis of IPF. The reported incidence ranges from 2% to 7%. The mechanism by which surgical manipulation of the lung could cause an exacerbation is currently speculative. Mortality associated with postoperative acute exacerbations is high, ranging from 50% to 100%. Steroids and other immunosuppressive agents are commonly initiated but there is no compelling evidence for efficacy. Lung transplantation may be an option in selected cases.
 PHRENIC NERVE INJURY AND DIAPHRAGMATIC DYSFUNCTION
PHRENIC NERVE INJURY AND DIAPHRAGMATIC DYSFUNCTION
Phrenic nerve injury is a well-described complication of CABG. In the past, this complication arose chiefly from the use of iced saline slush placed in the pericardium for topical cooling of the heart. Thermal injury causes both demyelination and axonal degeneration of the nerve, with slowing of conduction and impaired activation of the diaphragm. The use of topical cooling techniques has fallen out of favor largely because of this potential complication. However, the phrenic nerves can also be injured by traction, ischemia, use of diathermy, or transection during sternal retraction and harvesting of the internal mammary arteries. Unilateral phrenic nerve injury, typically involving the left phrenic, has been reported in approximately 10% of patients undergoing CABG.41,81 Bilateral phrenic nerve injury was reported to occur in 1% to 3% of cases in the era of widespread topical cardioplegia usage but is now a rare event. Phrenic nerve injury is not restricted to CABG but is also seen in association with other cardiac procedures, thoracic surgery, neck surgery, and liver transplantation.
Although typically inconsequential in the otherwise healthy patient, unilateral diaphragmatic paralysis can lead to significant respiratory compromise in patients with underlying chronic lung disease or those who are otherwise marginal. In patients with COPD, for example, the duration of postoperative mechanical ventilation and the rate of reintubation are higher for those with versus without unilateral phrenic nerve injury following CABG.82 Bilateral diaphragmatic paralysis results in marked impairment in pulmonary function and frequently leads to respiratory failure. In the proper setting, phrenic nerve injury should be suspected when attempts to wean a postoperative patient from mechanical ventilation result in progressive hypercapnia or atelectasis. The spontaneously breathing patient will often complain of orthopnea, which may be misinterpreted by the unsuspecting clinician as indicative of congestive heart failure. However, orthopnea is actually due to further impairment in diaphragmatic function resulting from loss of gravitational assistance in the supine position. The detection of inspiratory thoracoabdominal paradox – an inward movement of the abdominal wall with simultaneous expansion of the thorax – is an important bedside clue to the presence of bilateral diaphragmatic paralysis and is best evoked in the supine position. The chest radiograph may also hold important clues, demonstrating either unilateral or bilateral elevation of the diaphragms and accompanying basilar atelectasis. However, these findings are not specific for phrenic nerve injury and may also be due to splinting or abdominal distention. A reduced maximum inspiratory pressure recorded at the mouth is another sensitive but nonspecific indication of significant diaphragmatic dysfunction.
Unilateral diaphragmatic paralysis can be readily diagnosed by fluoroscopic inspection, which reveals paradoxical upward movement of the affected hemidiaphragm with a maximal inspiratory effort (“sniff”). The situation is more problematic with bilateral diaphragmatic dysfunction. In this setting, patients often assume an altered breathing pattern marked by active contraction of the abdominal muscles during expiration, forcing the flaccid hemidiaphragms upward. With subsequent inspiration, the abdominal muscles relax and the hemidiaphragms descend briefly, potentially creating the false impression that they are functional. Because of this, fluoroscopy may not be confirmatory in these patients. Ultrasound has emerged as a simple bedside test for assessing diaphragmatic function. Thickening of the diaphragm with inspiration reflects diaphragmatic shortening and failure to observe this sign is indicative of diaphragmatic paralysis. Ultrasound can thus be used to diagnose both unilateral and bilateral paralysis and can be performed serially to assess for recovery of function.83
The gold standard for confirmation of phrenic nerve injury is electrophysiological testing, although even this methodology is occasionally flawed. The phrenic nerve is stimulated transcutaneously in the neck, and the diaphragmatic electromyogram (EMG) is recorded by surface electrodes placed in the seventh intercostal space at the costochondral junction. Demonstration of a prolonged latency between nerve stimulation and diaphragmatic action potential confirms a diagnosis of demyelinating injury. It is more difficult to interpret the significance of diminished amplitude or complete absence of the surface recording of the diaphragmatic EMG. This finding could represent either phrenic nerve injury/transection or failure to properly localize the diaphragm, which is typically shifted caudally in the postoperative patient and, therefore, away from the surface electrodes. Direct puncture of the diaphragm with a recording electrode may be employed to clarify this issue, but the technique requires a high level of expertise and carries a risk of pneumothorax.
Nontraumatic causes of phrenic nerve injury and diaphragmatic dysfunction can also lead to prolonged respiratory failure and delayed weaning in the surgical patient. Phrenic neuropathy can be a component of a more generalized polyneuropathy of critical illness, commonly encountered in the wake of an episode of severe sepsis or systemic inflammatory response syndrome. A critical illness myopathy affecting the diaphragms and other muscles of respiration can be encountered under the same circumstances. Finally, diaphragmatic dysfunction can arise as a component of a myopathy induced by the concurrent use of high-dose systemic corticosteroids and neuromuscular blocking agents.
Patients with diaphragmatic dysfunction are generally well suited for noninvasive positive pressure ventilatory support if they are awake and able to effectively handle respiratory secretions. Tracheostomy is indicated for patients with ineffective cough and those who cannot be weaned from conventional mechanical ventilation. The prognosis for patients with thermal or traction injury of the phrenic nerve is favorable; recovery is typically complete, but often protracted. In symptomatic patients with unilateral diaphragmatic paralysis due to transection of the phrenic nerve, surgical placation of the flaccid hemidiaphragm may lead to improved pulmonary function and successful liberation from mechanical ventilation.84
 PULMONARY EMBOLISM
PULMONARY EMBOLISM
An increased risk of PE accompanies a number of surgical procedures, including upper abdominal, neurosurgical, cardiac, major urological, and lower extremity orthopedic procedures. Other, nonsurgical risk factors that predispose the patient to PE may also be present, including obesity, immobility, and underlying malignancy.
While alterations in gas exchange typify PE, frank hypoxemic respiratory failure is relatively uncommon and suggests massive clot burden. Lesser degrees of clot burden may produce equally devastating physiological impairment in patients with underlying pulmonary disease. In the presence of severe hypoxemia, there is little remaining cardiopulmonary reserve. Failure to establish a correct diagnosis and to swiftly and appropriately intervene can prove lethal.
Unfortunately, little information pointing specifically to a diagnosis of PE is easily gleaned at the bedside. The patient is often dyspneic, and tachypnea and tachycardia are observed on physical examination. However, these features are common in many postoperative patients because of pain and atelectasis. More informative, but infrequently detected, is evidence of acute cor pulmonale: distended neck veins, a parasternal heave, right-sided third heart sound, and accentuation of the pulmonic component of the second heart sound. An electrocardiogram may also demonstrate evidence of right heart strain, with an “S1Q3T3” pattern or new right bundle branch block. The chest radiograph is most suggestive of PE when it is normal in the face of severe hypoxemia. When abnormal, the greatest utility of the chest radiograph is in identifying other causes of hypoxemia such as pneumonia, pneumothorax, or ARDS. Echocardiography is commonly performed in the setting of hypotension; evidence of a dilated right ventricle in the face of a normal or underfilled left ventricle should raise suspicion for massive PE.
CT angiography has emerged as the imaging procedure of choice, assuming the patient is sufficiently stable to be safely transported for the study. The accuracy of CT angiography in diagnosing acute PE was most comprehensively assessed in the Prospective Investigation of Pulmonary Embolism Diagnosis II (PIOPED II) trial.85 The positive predictive values of this technique were documented to be 96% and 92% when coupled with a high or intermediate clinical suspicion for PE, respectively, as assessed by use of the Wells Score. Conversely, the negative predictive value was 96% when coupled with a low clinical suspicion. Notably, discordance of radiographic results with clinical assessment significantly compromised the performance of CT angiography. For example, 42% of CT angiography readings were falsely positive among patients with a low clinical probability of PE. Conventional pulmonary angiography represents an alternative diagnostic modality in situations where CT angiography is not definitive.
While anticoagulation with heparin forms the mainstay of therapy for the otherwise stable patient, the presence of life-threatening hypoxemia and/or hemodynamic instability should prompt consideration of alternative or additional interventions. Since additional clot burden could be fatal, insertion of an inferior vena cava filter is generally advised in this setting and should be considered mandatory when anticoagulation is contraindicated. Thrombolytic therapy should also be considered in the critically ill patient, but its use in the postoperative period is limited by the risk of precipitating bleeding at the site of recent surgery. This risk appears to fall to an acceptable level beyond the seventh postoperative day; the exception is intracranial surgery, which contraindicates use of lytic agents for at least 2 months. Several interventional radiological techniques – thrombus fragmentation, suction embolectomy, and intraembolic infusion of low-dose thrombolytics – as well as surgical embolectomy are alternative considerations in the deteriorating patient for whom systemic thrombolytics are either contraindicated or unsuccessful.
 OBSTRUCTIVE SLEEP APNEA
OBSTRUCTIVE SLEEP APNEA
Obstructive sleep apnea (OSA) is highly prevalent within surgical populations, affecting approximately 25% of adults undergoing elective general surgical procedures,86 over 30% of neurosurgical patients,87 and greater than 70% of morbidly obese patients undergoing bariatric surgery.88 It is characterized by repetitive upper airway obstruction during sleep, resulting in arterial desaturation, hypercapnia, and arrhythmias. The perioperative period is a particularly precarious time for patients with this disorder. Because of alterations in oropharyngeal anatomy that commonly accompany obesity and OSA, orotracheal intubation at the time of induction may be difficult. The use of anesthetics, opioids, and sedatives diminishes the activity of the upper airway dilator musculature and concurrently dampens respiratory arousal mechanisms that can terminate obstructive apneas. These effects lead to an increase in the frequency and duration of obstructive apneas that, in turn, can place the surgical patient at significant risk for serious respiratory complications. A recent meta-analysis demonstrated that the incidence of postoperative respiratory failure was significantly higher among patients with OSA compared to those without.87 Similarly, analysis of a national database of approximately 6 million surgical patients demonstrated that OSA increased the risk of postoperative intubation and mechanical ventilation fivefold after orthopedic procedures and twofold after general surgical procedures.89
As many as 80% of patients with OSA are undiagnosed at the time of surgery.86 Identification of high-risk candidates with OSA prior to surgery is thus an essential strategy in minimizing the adverse impact of OSA on postoperative outcomes. Several easily administered screening tools have been developed and validated that can be incorporated into the preoperative assessment of surgical candidates. For example, the STOP-Bang tool uses a self-administered four-item questionnaire in conjunction with assessment of four patient characteristics (Table 104-5). It has positive predictive and negative predictive values of 52% and 90%, respectively, in identifying surgical candidates with moderate-to-severe OSA (apnea–hypopnea index >15).90



