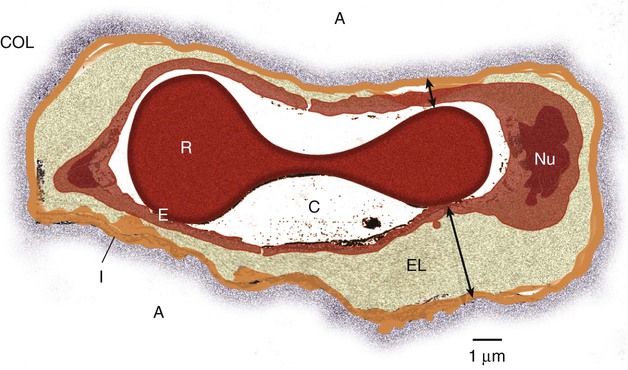
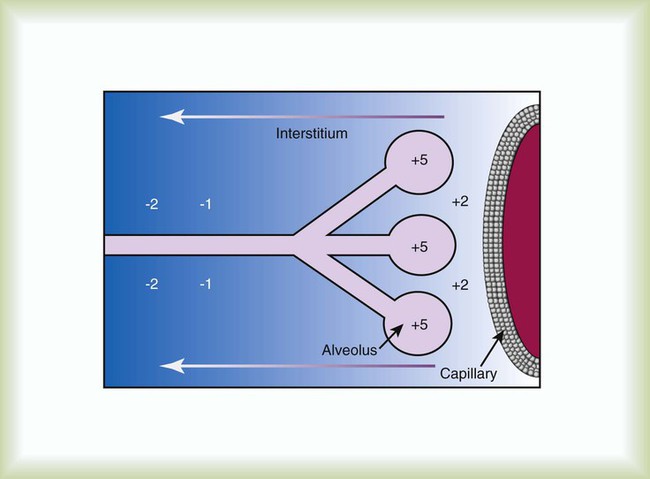
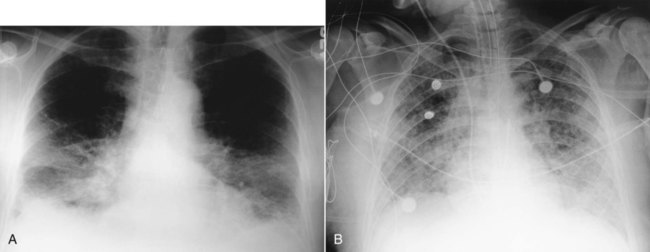
After reading this chapter you will be able to: Acute hypoxemic respiratory failure may develop in many clinical settings and is a common reason for admission to the intensive care unit (ICU). Most cases of acute hypoxemic respiratory failure develop as a result of abnormal accumulations of fluid within the lung parenchyma and alveoli. These accumulations are collectively referred to as pulmonary edema. Pulmonary edema may arise from acute illnesses associated with increased pulmonary venous pressure (hydrostatic pulmonary edema or congestive heart failure [CHF]) or may result from conditions associated with acute lung injury (ALI), in which the normal barriers to fluid movement within the lungs are disrupted (nonhydrostatic pulmonary edema). ALI of sufficient severity to cause acute hypoxemic respiratory failure is commonly referred to as acute respiratory distress syndrome (ARDS). ALI and ARDS represent a spectrum of lung injury with many patients initially presenting with ALI and progressing to ARDS with more severe gas exchange abnormalities (Table 27-1).1,2 TABLE 27-1 Recommended Criteria for Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS) Modified from Bernard GR, Artigas A, Brigham KL, et al: The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 49(3 Pt 1):818–824, 1994. ARDS is a common cause of respiratory failure. It can occur as a consequence of critical illnesses of diverse causes. The exact incidence of ARDS varies depending on the population being studied, but in the United States it is thought to range from 13.5 to 64 cases per 100,000 person-years.3,4 ARDS may be present in 16% of mechanically ventilated patients on admission to the ICU.2 Despite uncertainties regarding the incidence of ARDS, the mortality rate associated with ARDS has seemed to decline over the past 3 decades from more than 90% to the present level of 30% to 40%.5,6 It has been proposed that ARDS can develop via different mechanisms and that the risk factors for ARDS should be categorized as either direct injury via damage directly to the alveolar space or indirect injury initiated by systemic disease (Box 27-1).1 However, all of these risk factors share the common ability to initiate a systemic inflammatory reaction, which, if sufficiently vigorous, may lead to diffuse lung injury (ARDS). In this regard, the probability of developing ARDS may depend in part on the severity and characteristics of the initial injury. Gastric aspiration and septic shock (sepsis with refractory hypotension) are associated with a greater than 25% risk of ARDS, whereas the administration of multiple blood transfusions is associated with an ARDS risk of less than 5%.7 The risk of ARDS seems to be additive when multiple risk factors are present.8 The lung structure is optimally designed to fulfill its physiologic functions. These are as follows: • To deliver inhaled oxygen (O2) to the site of gas exchange—the alveoli • To diffuse gases, mainly O2 and carbon dioxide (CO2), between the alveolar capillary membrane and the lumen of the alveolus • To match alveolar ventilation with pulmonary capillary blood flow so that gas exchange is optimized • To maintain a net flux of fluid through the lung parenchyma without inducing lung edema or alveolar consolidation • To provide a barrier against toxic environmental exposures, including infectious agents, dusts, and fumes The intricate design of the pulmonary circulation provides for the efficient transfer of gases between the alveoli and the blood. On average, the entire blood volume of the body circulates through the lungs in 1 minute or less. This incredible feat is achieved through an ingenious design. Starting at the outflow tract of the right ventricle (pulmonary valve), the relatively thick-walled, smooth muscle–lined pulmonary artery branches successively and follows the divisions of the bronchi as far as the terminal bronchioles. Beyond the terminal bronchioles, the pulmonary vasculature divides further to form a fine capillary meshwork surrounding the alveoli. The large surface area of the capillary network provides for a low-pressure (5 to 12 mm Hg), high-volume system wherein large volumes of blood come into immediate contact with alveolar gases. At a capillary level, the vessel walls are composed solely of endothelial cells bound to a basal lamina. Because of the delicate nature of the alveolar-capillary interface, injury to the alveolar-capillary interface and high capillary blood pressure result in disruption of pulmonary gas exchange (see later section on Pulmonary Edema). The interstitial space of the lung is the space between the alveolar epithelium and the capillaries. The interstitium is composed of several structural proteins (types I, III, and IV collagen and elastin) and proteoglycans. The proteoglycans make up the ground substance of the interstitium and are composed of 20% protein and 80% glycosaminoglycans. The alveolar-capillary interstitial space is composed of endothelial and epithelial cell membranes bound to a common basement membrane with a very thin (<0.5 µ) interstitial space. In contrast, the interstitium on the nonalveolar side of the capillaries contains separate basement membranes for both epithelial cells and endothelial cells and fibroblasts, structural collagen, elastin proteins, and mucopolysaccharides in a hyaluronic acid gel (Figure 27-1). The lung protects itself from the devastating consequences of excessive fluid accumulation by several mechanisms. The lung lymphatic drainage system is the primary operant system under nonpathologic conditions. The lymphatic drainage system is the main conduit for the removal of filtered fluid and protein from the lungs. Fluid and solutes enter the lymphatic drainage channels from small lymphatic capillaries located around the respiratory bronchioles. This process is assisted by the presence of a modest pressure gradient within the lungs. That is, pressure is greatest within the dense alveolar interstitium and gradually decreases in the nonalveolar interstitium and terminal lymphatic vessels (Figure 27-2). Drainage is enhanced further by intrathoracic pressure alterations that occur with respiration, and retrograde flow is prevented by the presence of one-way lymphatic valves. Ultimately, lymphatic fluid drains into the superior vena cava through the thoracic duct.9 When fluid filtration exceeds the capacity for drainage through pulmonary lymphatic vessels, several “backup” systems exist for storing additional fluid and for protection against alveolar flooding. Loose connective tissue located along the peribronchovascular space and extending to the level of the bronchiole is capable of storing twice the normal fluid content of the lungs.9 Filling of these spaces or cuffs manifests radiographically as increased interstitial infiltrates (Figure 27-3), or Kerley’s lines, which are caused by increased fluid in interlobular septal spaces. The peribronchovascular spaces drain into the local blood vessels or follow the intrinsic pressure gradient in the lung and empty into pulmonary lymphatic vessels. As total lung fluid accumulation increases further, the gel-like matrix of the lung is capable of absorbing additional fluid without affecting interstitial pressure. The latter property is important because fluid is allowed to accumulate in the lungs without transmitting additional hydrostatic pressure to the alveolar barrier. Additional fluid movement into the lungs is avoided. The dense connective tissue making up the alveolar matrix resists edema formation in response to elevated hydrostatic or oncotic pressure. Hydrostatic pulmonary edema, also called cardiogenic pulmonary edema, is the result of increased venous hydrostatic pressure leading to fluid accumulation and alveolar flooding. The alveolar barrier is composed of the dense alveolar matrix (described earlier), the epithelial basement membrane, and the lining epithelial cells. The alveolar basement membrane is selectively permeable to only very small solutes such that osmotic forces favor the retention of fluid in the intravascular space. Alveolar pressure generally is slightly higher than interstitial pressure, and this difference provides further protection against alveolar fluid accumulation (see Figure 27-2). Under normal circumstances, there is little fluid movement into the alveoli across the alveolar barrier. Fluid that does form is composed of low-molecular-weight proteins and is actively transported back into the interstitium by type II pneumocytes.10 In contrast, when the vascular hydrostatic pressure increases and overwhelms the defense mechanisms against fluid accumulation in the lung, hydrostatic pressure increases sharply within the interstitium of the lung, and alveolar flooding ensues. The precise mechanism of alveolar flooding in hydrostatic pulmonary edema is unknown. It has been shown, however, that alveolar flooding occurs in an “all-or-none” manner. The alveolar fluid formed in the setting of increased hydrostatic pressure has characteristics identical to interstitial fluid even though the alveolar epithelium is normally impermeable to large proteins and molecules.9 This observation lends support to the findings of Conhaim,11 who showed experimentally that high interstitial fluid pressure leads to alveolar flooding through leakage of fluid from the epithelium of respiratory bronchioles, alveolar ducts, and their associated alveoli. The epithelium of the respiratory bronchioles and alveolar ducts may be particularly prone to hydrostatic injury because these locations represent the transition zone between respiratory and alveolar epithelium. Nonhydrostatic pulmonary edema, also called noncardiogenic pulmonary edema, is the result of the loss of microvascular membrane integrity. In contrast to hydrostatic pulmonary edema, nonhydrostatic pulmonary edema is associated with increased total lung water despite normal microvascular hydrostatic pressure. The mechanisms of nonhydrostatic pulmonary edema that ultimately lead to ARDS are more complex than the mechanisms responsible for hydrostatic pulmonary edema. Although many seemingly unrelated risk factors for ARDS have been identified, all causes of ARDS evoke disruption of endothelial and epithelial barriers and typically occur under conditions associated with widespread microvascular injury to the lungs. Vascular endothelial injury in the lungs results in increased microvascular permeability and fluid filtration, such that there is uninhibited entry of protein-rich fluid into the pulmonary interstitium. Alveolar flooding develops when the osmotic gradient between the capillary and the lung becomes essentially zero and no longer opposes the hydrostatic forces favoring fluid movement from the capillary into the lung: Kfc(Pmv − Pi) >> (sd)(TTmv − Tti) (see earlier equation). This process is likely facilitated both by damage to the normally impermeable alveolar epithelial barrier, which is a key feature of ARDS,12 and by impaired alveolar fluid clearance in ALI and ARDS.13 Investigations support a role for both necrotic epithelial cell death and programmed cell death (apoptosis) in the pathogenesis of alveolar wall damage.14,15 In addition, therapies aimed at improving the vascular barrier to fluid movement have shown protection against ALI animal models. A common mechanism to explain how different acute illnesses (e.g., sepsis, gastric acid aspiration, pancreatitis) can lead to the development of ARDS was proposed by Weiland and colleagues.16 These investigators showed that ARDS, regardless of the cause, is associated with an influx of polymorphonuclear neutrophils (PMNs) and PMN-derived inflammatory by-products, such as neutrophil elastase and myeloperoxidase, into the lung. The PMN-activating cytokine interleukin (IL)-8 has been shown to be increased in the lungs of patients with ARDS, whereas a reduction in IL-8 and PMNs has been shown to correlate with recovery from ARDS.17 Taken together, the results of these studies suggest that the common mechanism for the development of ARDS is induction of lung inflammation leading to loss of membrane integrity, as a result of either local lung injury (direct injury) or systemic inflammation (indirect injury). Although PMNs play a central role in the development of ARDS, other chemical (e.g., gastric aspiration) and immunologic pathways participate in the initiation and development of the systemic inflammatory response to critical illnesses associated with ARDS. Sepsis is associated with intense activation of systemic inflammatory pathways such that cytokines (e.g., tumor necrosis factor [TNF]-alpha, IL-1beta, IL-6, and IL-8), arachidonic acid metabolites (e.g., platelet-activating factor, leukotrienes), and nitric oxide (NO) all contribute to the hemodynamic and inflammatory events characteristic of this syndrome.18 The relative contributions and exact roles of these proinflammatory mediators in the pathogenesis of ARDS and MODS remain a topic of intense investigation and controversy.19,20 It is likely that there is no single dominant pathway in the development of ARDS because attempts to control the inflammatory response in sepsis by blocking specific mediators, such as anti-TNF antibodies, have not proved beneficial and may worsen outcome. ARDS is associated with restrictive physiology and refractory hypoxemia, which are largely a result of pulmonary microvascular injury. Specifically, increased pulmonary capillary permeability facilitates the influx of inflammatory fluid into the lung interstitium and alveolar spaces and causes decreased lung compliance and alveolar consolidation. The presence of intraalveolar inflammatory fluid impairs surfactant synthesis and function so that pulmonary gas exchange (i.e., related to atelectasis) and compliance are further impaired. The negative effects of alveolar consolidation and atelectasis on pulmonary gas exchange are exacerbated by a loss of the normal vascular response to alveolar hypoxemia. The body is unable to shunt blood away from the diseased alveoli, and these unaerated alveoli receive excessive blood flow, which contributes to severe ventilation/perfusion mismatching and an effective intrapulmonary right-to-left shunting of blood flow in ARDS. The pulmonary manifestations of ARDS and CHF are summarized in Box 27-2. The notion that factors operating outside the lungs may participate in the initiation and progression of ARDS has generated interest in the role of organ system interactions in the pathogenesis of ARDS and MODS. For example, injury to remote systemic organs is known to occur after ALI,21 apparently related to PMN-mediated mechanisms.22 In this way, ALI may perpetuate the systemic inflammatory response and lead to further lung and systemic organ injury. The gut-liver-lung axis may be most influential in causing the systemic inflammatory response associated with ARDS and MODS. The gastrointestinal (GI) tract contains large quantities of potentially pathogenic bacteria against which the host is normally protected by intact mucosal barriers and the reticuloendothelial system. However, the function of the GI tract and the liver is frequently compromised in critical illness. The widespread use of broad-spectrum antibiotics in the care of critically ill patients often leads to overgrowth of antibiotic-resistant bacteria within the GI tract. These bacteria and their toxic by-products (e.g., endotoxin) escape from the GI tract and are taken up by the reticuloendothelial cells of the liver, spleen, and regional lymph nodes. The resultant activation of the reticuloendothelial system may initiate and perpetuate a systemic inflammatory response that leads to systemic organ injury (i.e., ARDS and MODS).23 This sequence of events forms the basis for strategies designed to reduce the release of proinflammatory mediators from the GI tract, including selective decontamination, early enteral feeding, and other approaches designed to moderate the systemic inflammatory response in ARDS and MODS (see later section on Therapeutic Approach to Acute Respiratory Distress Syndrome). Why ARDS and MODS develop in some patients with ALI and not in others is unknown. The determinants of ARDS may relate to the balance between proinflammatory and antiinflammatory factors within the body. In this regard, the liver plays a major role in both induction and modulation of the systemic inflammatory response to all kinds of initiating events and is primarily responsible for the breakdown of endogenous proinflammatory mediators, including TNF-alpha, leukotrienes, and others.24 Patients with liver disease have higher levels of circulating proinflammatory mediators, are more prone to bacteremia, and have a high incidence of ARDS compared with patients without liver disease.25 The liver is not the sole determinant of ARDS and MODS in critically ill patients. Other factors, such as the severity of the primary illness and comorbid diseases (e.g., cardiac disease, advanced age, renal failure, malignant disease) and perhaps the genetic profile of the patient, also seem to predispose patients to ARDS and MODS.23,26 The exudative phase is characterized by diffuse damage to alveoli and blood vessels and the influx of inflammatory cells into the interstitium. Many of the alveolar spaces become filled with a proteinaceous, eosinophilic (on hematoxylin and eosin stain) material called hyaline membranes, which are composed of cellular debris and condensed plasma proteins. Pathologically, there is destruction of type I pneumocytes, which are normally the predominant cells lining the alveoli; type II pneumocytes are relatively resistant to injury.27,28 Patients with ARDS have profound dyspnea, tachypnea, and refractory hypoxemia. This phase of ARDS often is difficult to differentiate from respiratory failure related to hydrostatic pulmonary edema (CHF). The clinical presentations of these two forms of acute respiratory failure are discussed later (see the section on Differentiating Hydrostatic from Nonhydrostatic Pulmonary Edema in the Clinical Setting). The exudative phase may be self-limited or may progress to a fibroproliferative phase. After inflammatory injury to the lung is established and the initiating events are controlled, a process of lung repair begins. Pathologically, there is hyperplasia of alveolar type II pneumocytes and proliferation of fibroblasts within the alveolar basement membrane and intraalveolar spaces. Fibroblasts mediate the formation of intraalveolar and interstitial fibrosis.27 The extent of fibrosis determines the degree of pulmonary disability in patients who survive ARDS. The exact mechanisms controlling lung remodeling in ALI are not well established but very likely involve by-products of inflammatory cells (e.g., proteases, antiproteases, IL-6) and various growth factors (transforming growth factor [TGF]-alpha, TGF-beta).29,30 However, the remodeling process after ARDS is quite variable. In some cases, patients have nearly complete normalization of lung compliance and oxygenation for 6 to 12 months after the illness. In other cases, the architecture of the lung never returns to normal, and patients experience severe respiratory disability related to extensive pulmonary fibrosis and obliteration of the pulmonary vasculature. The pattern of fibrosis after ALI suggests that, as in repair of the skin, an intact basement membrane is necessary for normal repair of the epithelium of the alveoli. It follows that disruption of the alveolar basement membrane is a prerequisite to the development of fibrosis after ALI. This line of reasoning is supported by the observation that the extent of recovery depends on the severity of the initial lung injury and on the influence of secondary forms of injury. Secondary forms of lung injury include nosocomial infection, O2 toxicity, and barotrauma (see later section on Therapeutic Approach to Acute Respiratory Distress Syndrome).
Acute Lung Injury, Pulmonary Edema, and Multiple System Organ Failure
 Identify the approximate incidence rate of acute respiratory distress syndrome (ARDS) and how the mortality rate has changed over the past several decades.
Identify the approximate incidence rate of acute respiratory distress syndrome (ARDS) and how the mortality rate has changed over the past several decades.
 State the risk factors associated with the onset of ARDS.
State the risk factors associated with the onset of ARDS.
 Describe how the normal lung prevents fluid from collecting in the parenchyma and how these mechanisms can fail and cause pulmonary edema.
Describe how the normal lung prevents fluid from collecting in the parenchyma and how these mechanisms can fail and cause pulmonary edema.
 Describe the effect pulmonary edema has on lung function, including gas exchange and lung compliance.
Describe the effect pulmonary edema has on lung function, including gas exchange and lung compliance.
 Describe the relationship between multiple organ dysfunction syndrome (MODS) and ARDS.
Describe the relationship between multiple organ dysfunction syndrome (MODS) and ARDS.
 Identify the histopathology associated with the exudative phase and the fibroproliferative phase of ARDS.
Identify the histopathology associated with the exudative phase and the fibroproliferative phase of ARDS.
 Differentiate hydrostatic and nonhydrostatic pulmonary edema based on clinical setting.
Differentiate hydrostatic and nonhydrostatic pulmonary edema based on clinical setting.
 Describe the principles of supportive care followed for patients with ARDS.
Describe the principles of supportive care followed for patients with ARDS.
 Describe how ventilator settings (e.g., tidal volume, positive end expiratory pressure, respiratory rate) are adjusted for patients with ARDS and MODS.
Describe how ventilator settings (e.g., tidal volume, positive end expiratory pressure, respiratory rate) are adjusted for patients with ARDS and MODS.
 Describe how mechanical ventilation can cause lung injury and how ventilator-induced lung injury can be avoided.
Describe how mechanical ventilation can cause lung injury and how ventilator-induced lung injury can be avoided.
 State the approaches to the management of ARDS and MODS.
State the approaches to the management of ARDS and MODS.
 Describe the use of innovative mechanical ventilation strategies in the support of patients with ARDS.
Describe the use of innovative mechanical ventilation strategies in the support of patients with ARDS.
 State the effect of prone positioning on oxygenation and mortality in a patient with ARDS.
State the effect of prone positioning on oxygenation and mortality in a patient with ARDS.
 Describe the value of pharmacologic therapies such as nitric oxide and corticosteroids in the treatment of patients with ARDS.
Describe the value of pharmacologic therapies such as nitric oxide and corticosteroids in the treatment of patients with ARDS.
Criteria Pressure
Timing
Oxygenation
Chest Radiograph
Pulmonary Artery Wedge
ALI
Acute onset
PaO2/FiO2 ≤ 300 mm Hg (regardless of PEEP level)
Bilateral infiltrates seen on frontal chest radiograph
≤18 mm Hg when measured or no clinical evidence of left atrial hypertension
ARDS
Acute onset
PaO2/FiO2 ≤ 200 mm Hg (regardless of PEEP level)
Bilateral infiltrates seen on frontal chest radiograph
≤18 mm Hg when measured or no clinical evidence of left atrial hypertension

Epidemiology
Risk Factors For Acute Respiratory Distress Syndrome
Pathophysiology
Normal Physiology
Pulmonary Blood Flow
Lung Interstitium
Liquid and Solute Transport in the Lungs
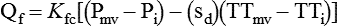
Pulmonary Edema
Hydrostatic Pulmonary Edema
Nonhydrostatic Pulmonary Edema
Gas Exchange and Lung Mechanics in Acute Respiratory Distress Syndrome
Role of Organ-Organ Interactions in the Pathogenesis of Acute Respiratory Distress Syndrome and Multiple Organ Dysfunction Syndrome
Histopathology And Clinical Correlates Of Acute Respiratory Distress Syndrome
Exudative Phase (1 to 3 Days)
Fibroproliferative Phase (3 to 7 Days)
Acute Lung Injury, Pulmonary Edema, and Multiple System Organ Failure