LEARNING OBJECTIVES
Learning Objectives
The student will be able to describe the definition, prevalence, and impact of ALI and ARDS in multidisciplinary critical care medicine.
The student will be able to list and define the direct and indirect risk factors for ALI/ARDS, and distinguish the clinical features of ALI and ARDS.
The student will be able to summarize the initiating pathophysiology of ALI/ARDS, and factors that perpetuate or are associated with its nonresolution, especially ventilator-associated lung injury.
The student will be able to explain and defend current guidelines for the treatment and supportive care of patients with ALI/ARDS.
Acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) represent a spectrum of respiratory failure of rapid onset characterized by diffuse, bilateral lung injury and severe hypoxemia that are caused by noncardiogenic pulmonary edema. ALI/ARDS can affect patients of any age or preexisting condition, although both predispose to worse outcomes. Respiratory failure may be initiated by pulmonary or extrapulmonary insults that increase alveolar epithelial and endothelial permeability, flood alveoli, and reduce lung compliance in a pattern reflecting an acute restrictive lung disease. Despite numerous prospective, double-blind clinical trials in patients with ALI/ARDS, the only treatment that improves survival is mechanical ventilation using a lung protective strategy in which tidal volume VT is carefully titrated to ~6 mL/kg of predicted body weight. Positive end-expiratory pressure (PEEP) ventilation is useful for alveolar recruitment in lungs that will be prone to atelectasis. Clinical vigilance must be comprehensive and anticipatory to guard against development of ventilator-associated pneumonia or worsened ALI. Although mortality can exceed 50%, survivors have a good prognosis for recovery of lung function.
GENERAL FEATURES OF ALI/ARDS
ALI and ARDS affect over 190,000 patients annually in the United States and cause 75,000 deaths. They occur rapidly and show diffuse, bilateral lung injury readily evident by x-ray or CT. Patients exhibit severe hypoxemia despite use of supplemental O2, and are considered emblematic of a noncardiogenic pulmonary edema with low alveolar ventilation/perfusion ratios (V̇A/ Q̇) and abnormal physiological shunting of O2. Such acute respiratory failure usually occurs with neutrophil (PMN)-mediated lung inflammation. Pathological increases in the permeability of alveolar epithelial and endothelial cells lead to dyspnea, tachypnea, and arterial hypoxemia. These permeability defects increase efflux of proteins and fluids from blood into the alveolar interstitium and airspaces, while alveolar liquid clearance mechanisms are impaired that normally would resolve the edema. Consequently, alveolar O2 exchange deteriorates.
ALI/ARDS is complex and multiphasic. The initiating factors that may cause early lung dysfunction may differ from those that perpetuate, complicate, or delay its resolution. ALI/ARDS can be caused by pulmonary-specific mechanisms, notably severe pneumonia, aspiration of gastric contents, embolism of fat, air, or amniotic fluid, chest trauma, near-drowning, and inhalation of noxious gases. ALI/ARDS can also occur due to systemic conditions, notably bacteremic sepsis, peritonitis, pancreatitis, hemorrhagic shock, burns, massive transfusions, and drug overdoses. Such indirect factors reflect pulmonary manifestations of acute systemic inflammation and generalized endothelial injury that may involve many organ systems. Approximately 15% of deaths from ALI/ARDS are due to progressive respiratory failure with intractable hypoxemia, increased dead space ventilation, and hypercarbia. The main cause underlying death in ALI/ARDS (~75% patients) is the multiple organ dysfunction syndrome (MODS) involving the heart, kidneys, coagulation system, and liver as well as lung.
CLINICAL DEFINITIONS OF ALI/ARDS
In 1994, the American-European Consensus Conference on ARDS issued definitions that have been widely adopted by clinicians and researchers. These include:
Acute lung injury. A syndrome of acute and persistent lung inflammation with increased vascular permeability characterized by:
acute onset (within days of exposure to predisposing direct or indirect causes);
diffuse bilateral infiltrates on x-ray consistent with noncardiogenic edema;
Pao2/FIo2 ≤ 300 mm Hg, regardless of the level of PEEP during mechanical ventilation. The ratio Pao2/FIo2 estimates lung oxygenation efficiency across the range of supplemental O2 provided to patients. Pao2 is measured in mm Hg and FIo2 is a decimal between 0.00 and 1.00. For example, in a subject with Pao2 = 100 mm Hg on room air (FIo2 = 0.21), the Pao2/FIo2 = 100/0.21 = 476 mm Hg;
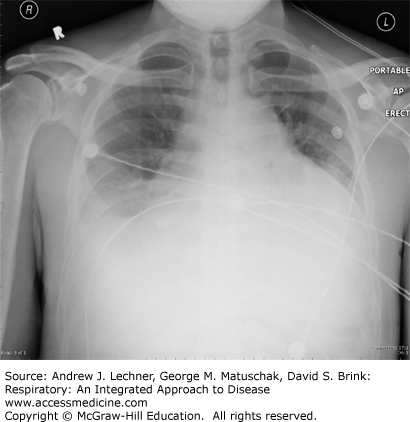
no clinical evidence of left-sided heart failure or elevated left atrial pressure, that is, an elevated PPW if a pulmonary artery catheter (PAC) had been placed, as in congestive heart failure (Fig. 28.1). A relatively normal heart size by x-ray supports the clinical absence of left ventricular failure as the explanation for pulmonary edema. If a PAC is in use, the wedge pressure is ≤18 mm Hg. Clinical evidence of left heart failure includes systolic left ventricular (LV) dysfunction, plus appropriate physical findings, such as LV S3 gallop and peripheral edema, especially when supported by LV dilation or reduced ejection fraction by echocardiography or catheterization, or enlarged cardiac silhouette plus pleural effusions.
Acute respiratory distress syndrome. A more severe physiological expression of ALI, at the further end of a spectrum of evolving lung injury characterized by:
acute onset;
diffuse bilateral infiltrates on x-ray consistent with pulmonary edema;
Pao2/FIo2 ≤200 mm Hg;
no evidence of left-sided heart failure, including normal heart size on x-ray (Fig. 28.2). If a PAC is used, the measured PPW is ≤18 mm Hg.
PATHOPHYSIOLOGY OF ALI/ARDS
Normal lung function requires dry, open alveoli overlying perfused capillaries with intact gas exchange and a normal work of breathing. It requires coordinated interaction of several key physiological processes plus appropriate lung immune and inflammatory responses. Three interrelated pathophysiologic abnormalities typify events that cause or precipitate ALI/ARDS.
1. Increased microvascular permeability (noncardiogenic pulmonary edema)
Normal pulmonary capillaries are selectively permeable, with serum proteins confined to intravascular spaces, while smaller molecules and water cross endothelial membranes by hydrostatic and osmotic forces. As discussed in Chap. 7, the Starling’s equation describes forces directing fluid movement between the vessels and the interstitium:
At least four mechanisms promote fluid retention in capillaries to prevent interstitial edema and alveolar flooding. First, airspace liquid is cleared by apical Na+ transporters in alveolar epithelial cells. Second, larger plasma proteins like albumin maintain osmotic gradients favoring water reabsorption. Third, tight junctions between pulmonary endothelial cells prevent leakage. Fourth, interstitial lymphatics return alveolar fluid to the circulation. Abnormalities in Starling’s forces during ALI/ARDS include:
Decreased σ during sepsis, causing larger proteins like albumin to enter the interstitium, so that protein-rich edema floods alveoli.
Reduced πMV from excessive IV infusions and/or reduced acute phase protein synthesis, favoring increased transvascular fluid flux into distal airspaces.
Increased PMV from IV fluids to treat hypovolemia, or decreased venous return due to increased intrathoracic pressures during positive-pressure ventilation.
Increased πPMV from plasma proteins entering alveolar interstitium, or from loss of alveolar epithelial Na+ transport, both retarding alveolar liquid clearance.
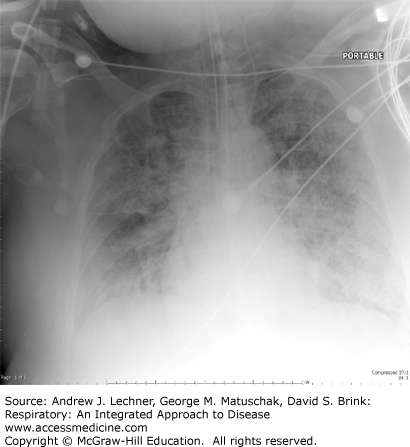
These cause gravitationally dependent alveolar edema, first evident in inferior lung zones of supine patients (Fig. 28.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree