Acute Lung Injury and the Acute Respiratory Distress Syndrome: Clinical Features, Management, and Outcomes
DESCRIPTION AND DEFINITIONS
In 1967, Ashbaugh et al.1 described a syndrome characterized by the acute onset of dyspnea, severe hypoxemia, diffuse lung infiltrates, and decreased respiratory system compliance in the absence of evidence for congestive heart failure (CHF). The syndrome, initially called acute respiratory distress in adults (to contrast it with acute respiratory distress in neonates), is now known as the acute respiratory distress syndrome (ARDS). Following the initial report, other authors utilized various definitions that incorporated elements related to time of onset, presence of hypoxemia and radiographic infiltrates, and absence of overt CHF.
In 1988, Murray and others introduced the Lung Injury Score (LIS), an assessment tool for ARDS that reflects the extent of radiographic infiltrates, severity of hypoxemia and reduced respiratory system compliance, and level of positive end expiratory pressure (PEEP) used in mechanically ventilating affected patients.2 The LIS incorporates four parameters that are graded on a scale of 0 to 4: (1) ratio of PaO2 to FIO2(PaO2/FIO2); (2) total respiratory compliance; (3) level of PEEP; and (4) extent of radiographic infiltrates (assessed by noting the number of quadrants in the chest radiograph demonstrating infiltrates). The LIS equals the sum of the scores for the four variables divided by four. In clinical studies, a score of 2.5 or more is generally used as a threshold for severe disease.
 CONSENSUS DEFINITIONS OF ACUTE LUNG INJURY AND ACUTE RESPIRATORY DISTRESS SYNDROME
CONSENSUS DEFINITIONS OF ACUTE LUNG INJURY AND ACUTE RESPIRATORY DISTRESS SYNDROME
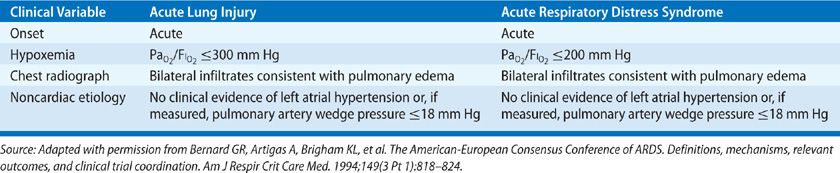
Prior to 1994, published studies used nonuniform definitions of ARDS, prompting an American European Consensus Conference (AECC) to develop standardized definitions for ARDS and acute lung injury (ALI)—a broader category that encompasses ARDS.3 The AECC definitions included the acute onset of illness, bilateral chest radiographic infiltrates consistent with pulmonary edema, poor systemic oxygenation, and absence of evidence for left atrial hypertension (Table 141-1A). The syndrome was referred to as ALI when the ratio of PaO2 to FIO2 (PaO2/FIO2); was ≤300, and ARDS when the ratio was ≤200. The AECC definitions of ALI and ARDS were intentionally broad in order to encompass different types of acute hypoxemic respiratory failure occurring in a wide variety of settings.
TABLE 141-1A American European Consensus Conference Criteria for Acute Lung Injury (ALI) and the Acute Respiratory Distress Syndrome (ARDS)
 DEVELOPMENT OF THE BERLIN DEFINITION OF ARDS
DEVELOPMENT OF THE BERLIN DEFINITION OF ARDS
The standardization of definitions of ALI and ARDS lead to significant advances in the understanding of the syndrome and allowed researchers and clinicians to communicate findings using common criteria. The AECC definitions were widely used in clinical trials, including those that demonstrated therapeutic benefit.4,5 In addition, they added important validity to the definition, allowing clinicians to generalize trial results to clinical decisions involving their own patients. Despite this standardization, little data were available to support the reliability and validity of ARDS definitions. In fact, several components of the AECC definition have been recognized as problematic: (1) Acute onset is not defined within a specific time frame. (2) The chest radiograph is subject to variability in interpretation.6,7 (3) PaO2/FIO2 may vary according to ventilator parameters, for example, PEEP, and at extremes of FIO2. (4) Accuracy in excluding the presence of heart failure may be influenced by measurement methodology and timing, as discussed below.8
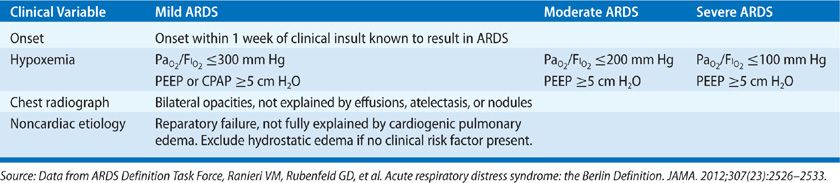
Given these limitations, an international expert panel was convened in Berlin in 2012 to revise the definition of ARDS. The resultant “Berlin definition” of ARDS addresses ambiguities in the prior AECC definition. The authors of the Berlin definition also applied proposed criteria for the syndrome to existing databases of patients with ARDS to specifically address the predictive validity of a new ARDS definition.9 According to the Berlin definition, ARDS is defined as a syndrome characterized by (1) onset within 1 week of a known clinical insult, (2) bilateral radiographic opacities, not fully explained by pleural effusions, atelectasis, or nodules, (3) respiratory failure not fully explained by cardiac failure or fluid overload, and (4) poor systemic oxygenation, with a PaO2/FIO2 ≤300 and a PEEP or CPAP ≥5 cm H2O. The definition also categorizes syndrome severity as mild, moderate, or severe, based on PaO2/FIO2. According to the Berlin definition, patients who previously were diagnosed with ALI, but not ARDS (i.e., PaO2/FIO2 ≤300, but >200), are referred to as having mild ARDS, with ALI now an obsolete term. Moderate ARDS is defined as PaO2/FIO2 ≤200, but >100, and severe ARDS is defined as PaO2/FIO2 ≤100 (Table 141-1B).
TABLE 141-1B Berlin Definition of Mild, Moderate, and Severe Acute Respiratory Distress Syndrome (ARDS)
While the Berlin definition leaves the AECC definition of ARDS largely unchanged, the new definition addresses several specific ambiguities of the prior diagnostic criteria. First, the definition defines “acute onset” and attempts to clarify chest radiograph criteria. In addition, the PaO2/FIO2 criterion is influenced by the level of PEEP and other transient factors, including the presence or absence of airway secretions or inadequate sedation. Increasing PEEP generally increases PaO2 at a given FIO2. The consequent increase in PaO2/FIO2 may result in a value that no longer meets criteria for ARDS. Conversely, without any PEEP, values of PaO2/FIO2 <300 may reflect simple atelectasis, rather than ARDS. Adding PEEP may recruit sufficient atelectatic lung to raise PaO2/FIO2 >300, thereby excluding such patients from meeting this criterion for ARDS. The Berlin definition addresses this concern directly by requiring a minimum level of PEEP when a determination of PaO2/FIO2 ratio is made.
Finally, given the declining use of pulmonary artery catheters (PACs),10 potential inaccuracies of pulmonary artery occlusion pressure (PAOP) measurements, and the now recognized possibility of concomitant hydrostatic edema and ARDS, the Berlin definition no longer specifies PAOP requirements.11,12 PAOP in ARDS may be higher than 18 mm Hg due to intravascular volume loading, particularly in the setting of application of goal-directed management paradigms for sepsis. Conversely, some patients with pulmonary edema due to CHF and high left atrial pressures have a normal PAOP by the time the catheter is inserted and PAOP is measured. The Berlin definition specifies that the respiratory failure of ARDS is not fully explained by cardiac failure or fluid overload and requires objective assessment to exclude hydrostatic edema (e.g., an echocardiogram) if no risk factor for ARDS is present.
The predictive validity of the new ARDS definition was assessed in several large cohorts of patients with ALI, as defined by AECC. Compared with the AECC definition, the Berlin definition had marginally better predictive validity for mortality. The identified categories of mild, moderate, and severe ARDS corresponded with progressive increases in mortality. Proposed ancillary variables in the definition of severe ARDS, including a chest radiograph with three or four quadrants demonstrating opacities, PEEP ≥10 cm H2O, and either a respiratory system static compliance ≤40 mL/cm H2O or a corrected expired volume per minute of at least 10 L/min did not improve the predictive validity of the definition of severe ARDS. Therefore, the final definition did not include any of these ancillary variables.
While the Berlin definition has been accepted by major professional organizations, it is important to note that the majority of the studies referred to in this chapter were performed prior to its development, and, therefore, utilize the older AECC definition of ALI and ARDS. Further refinements in the reliability and validity of definitions of ARDS constitute important future directions for clinical studies. In addition, attempts at understanding the heterogeneity underlying the syndrome may allow for identification of ARDS subgroups with potential diverse risk factors and pathogenesis. More reliable definitions will not only improve estimates of the public health impact of these syndromes, but also will decrease misclassification errors, which can be especially problematic for research aimed at clarifying underlying mechanisms.
EPIDEMIOLOGY
Over the last several decades, the epidemiology of ARDS has become more clearly delineated.
 INCIDENCE AND MORTALITY RATE
INCIDENCE AND MORTALITY RATE
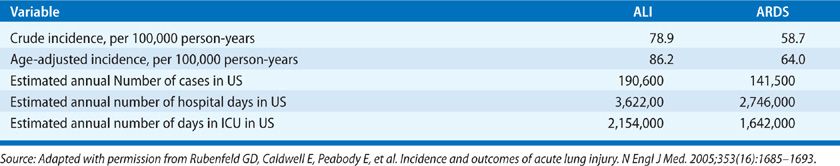
A landmark epidemiologic study of the incidence of ARDS in the United States between 1999 and 2000 – the King County Lung Injury Project (conducted in King County, Washington) – represents the first broad, population-based epidemiologic study of ARDS in the United States using the standardized AECC definitions.13 Study results included an estimated incidence of ARDS based on a PaO2/FIO2 ≤300 of 78.9 per 100,000 person-years and an age-adjusted incidence of 86.2 per 100,000 person-years. The incidence of what is now called moderate or severe ARDS was estimated as 58.7 per 100,000 person-years, with an age-adjusted incidence of 64.0 per 100,000 person-years. The incidence of ARDS increased dramatically with age, with an incidence of 306 per 100,000 person-years for ages 75 through 84 years. By extrapolation, an estimated 190,600 cases of ARDS occur each year in the United States (Table 141-2).
TABLE 141-2 Estimated Incidence, Hospital Days and Intensive Care Unit (ICU) Days for Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS) in the United States (US) based on American European Consensus Criteria for ALI and ARDS
Although prior estimates of the incidence of ARDS had been lower, the studies were limited by incomplete and nonvalidated data, inaccuracies in the definition of the syndrome, and use of administrative coding.14–19 Thus, the estimates from King County Lung Injury Project serve as the best indicator of the public health impact of ARDS in the United States. More recently, a retrospective cohort study of all patients admitted to the ICU at the Mayo Clinic from 2001 to 2008 has been reported.20 Since the Mayo Clinic provides all ICU-level care to the population of Olmsted County, Minnesota, the authors could estimate the incidence trends of ARDS over time. The age and sex adjusted incidence of moderate and severe ARDS (PaO2/FIO2 ≤200) declined from 81 cases per 100,000 person-years in 2001 to 38.3 cases per 100,000 person years in 2008. This trend appeared to be driven by declining hospital-acquired ARDS, perhaps the result of improvements is overall ICU care and the increasing use of lower tidal volume ventilation (see Approach to Treatment).
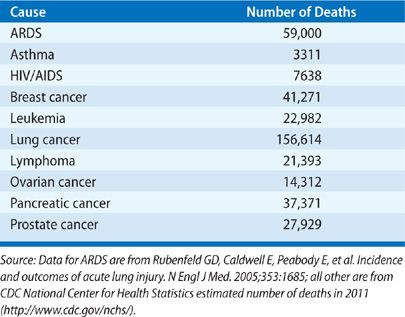
During the time of observation in the King County Lung Injury Project study, the mortality from ARDS was 38.5%, translating into an estimated 74,500 annual deaths from ARDS in the United States.13 Reported mortality estimates have ranged from 26% to 58%19–23 To put these figures into perspective, more people die annually with ARDS than from AIDS, asthma, and breast cancer combined.24 Other than lung cancer, ARDS leads to more annual deaths than any type of cancer, including lymphoma, leukemia, and breast, prostate, colon, ovarian, and pancreatic cancers (Table 141-3). The most common causes of death among patients with ARDS arise from the underlying insult or subsequent nosocomial pneumonia and sepsis.25,26 Only rarely do patients die from progressive hypoxia and respiratory failure.
Several studies have suggested that ARDS mortality has decreased over time.26–29 In one hospital using the same definition of ARDS throughout the period analyzed, the mortality rate was 68% in 1982, 29% in 1996, and in the mid-30% range in 1997 and 1998.26 Obviously, the decrease cannot be ascribed to widespread application of low tidal volume ventilation strategies, since the decline was observed prior to publication of the study reporting the value of using a “low stretch” ventilator protocol in 2000.4 The improvement is likely attributable to enhancements in general ICU care, including prevention of nosocomial pneumonias and other infections, earlier institution of enteral nutrition, routine use of stress ulcer prophylaxis, and improved teamwork in the ICU.
Interestingly, the case fatality rate for patients with ARDS due to sepsis remained the same over the same time frame noted earlier, while that for patients with ARDS due to trauma or other causes decreased significantly. Mortality rates among subjects enrolled in ARDS Network trials4,30–33 appear to have decreased from 35% (1996–1997) to 26% (2004–2005).27,34 These findings were robust in adjusting for covariates, including severity of illness and use of low tidal volume ventilation. However, these rates were obtained from clinical trials in specialized centers, excluding large numbers of patients with high-risk comorbidities. Recent systematic reviews of clinical trials and more inclusive prospective cohort studies have drawn opposing conclusions.28,35 Also, the previously noted study from the Mayo Clinic demonstrated a reduction in ARDS incidence, but it failed to show a change in mortality.20 While it is unclear if survival from ARDS is improving, the mortality rate remains unacceptably high and represents a major public health problem. Furthermore, as the population of the United States becomes older, the incidence of those at risk for ARDS and ARDS-related death can be expected to rise.
Precipitating Causes
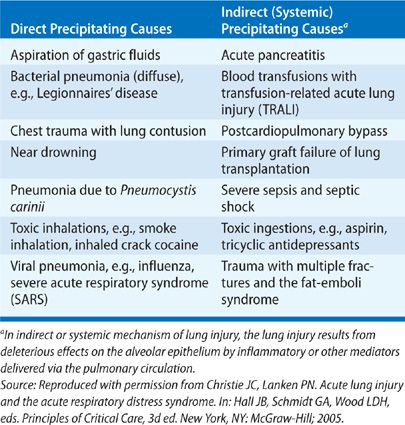
The severe, extensive lung inflammation characteristic of ARDS represents the common final pathogenic process in response to a large variety of precipitating causes,36–39 resulting in either direct or indirect (systemic) lung injury.40,41 In general, direct causes of ARDS include those that originate within the lung, such as aspiration of gastric contents or viral pneumonia. Examples of indirect causes include sepsis, ingested toxins, hypotension, and ischemia–reperfusion injury. Although some causes of ARDS may fit into either category (e.g., multilobar pneumonia with septic shock), this simple classification scheme is useful both for considering the many predisposing causes of ARDS and their varying mechanisms of lung injury and for future development of therapies aimed at different categories of ARDS. Table 141-4 lists precipitating causes of ARDS according to this construct.
 FACTORS INFLUENCING RISK OF ARDS
FACTORS INFLUENCING RISK OF ARDS
Not all patients with an underlying cause (e.g., sepsis) of ARDS develop the syndrome. In addition to inherent risk differences within at-risk populations, specific clinical variables may be important.
Clinical variables found to be associated with an increased risk of ARDS include chronic alcohol abuse,42 hypoproteinemia,43 advanced age,13 increased severity, and extent of injury or illness as measured by injury severity score (ISS) or APACHE score, hypertransfusion of blood products,38,44 and possibly, cigarette smoking.45,46 Diabetes mellitus and prehospitalization antiplatelet therapy appear to decrease the risk of ARDS.47–49 The role of obesity in modifying ARDS risk is complicated, as excess soft tissue can render chest x-ray interpretation difficult; the potential role of obesity remains the subject of ongoing research. A higher body mass index has been associated with an increased incidence of ARDS; however, obesity has not been associated with a consistent change in mortality.50–52 Since many studies of risk factors for ARDS are retrospective or based on a single center’s experience, the consistency and generalizability of identified risk factors have not been confirmed. Nonetheless, the mechanistic underpinnings and potential therapeutic implications of these probable associations are the subject of ongoing research.
 FACTORS INFLUENCING MORTALITY FROM ARDS
FACTORS INFLUENCING MORTALITY FROM ARDS
Clinical variables at the onset of ARDS that are associated with increased mortality include advanced age, lower PaO2/FIO2, high plateau pressure (i.e., low respiratory system compliance),53 greater extent of pulmonary infiltrates, sepsis, chronic liver disease, nonpulmonary organ dysfunction, increased global severity of illness, hypoproteinemia, and greater length of hospitalization prior to onset of ARDS.13,43,53–58 In addition, an increased dead-space fraction has been identified as a risk factor for increased mortality, possibly indicating the importance of early loss of the pulmonary vascular bed as a sign of greater disease severity.59,60 Although various precipitating causes of ARDS carry somewhat different prognoses, the strategy of low tidal volume ventilation utilized by the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI)–sponsored ARDS Clinical Trials Network (ARDSNet) as part of its clinical trials appears to be equally efficacious in all subgroups.4
 PREDICTING ARDS
PREDICTING ARDS
Using the identified precipitating causes and modifying risk factors, investigators have recently developed and validated a lung injury prediction score (LIPS) aimed at identifying those at highest risk of developing ARDS early.61,62 The LIPS was developed in a multicenter cohort of 5,584 hospitalized patients at risk for ARDS, among whom 7% eventually developed the syndrome. The LIPS is generated from the sum of points assigned for each of six predisposing conditions (shock, aspiration, sepsis, pneumonia, high-risk surgery, and high-risk trauma) and nine risk modifiers (alcohol abuse, obesity, hypoalbuminemia, chemotherapy, need for oxygen, tachypnea, hypoxia, academia, and diabetes mellitus). The negative predictive value for a score <4 was 97%, while the sensitivity and specificity for ARDS of a score >4 were 69% and 78%, respectively. While not perfect, the LIPS allows for the identification of a higher-risk population and may prove useful for studies of preventative interventions. The goal of identifying predictive and early indicators of ARDS is to shift the syndrome’s treatment paradigm to strategies aimed at prevention and treatment at the earliest phases of development.
CLINICAL PRESENTATION AND DIAGNOSIS
The clinical presentation and diagnosis of ARDS are fundamentally related to the syndrome’s pathophysiologic changes, regardless of the underlying etiology. A brief description of the pathology and pathophysiology is provided before a detailed discussion of clinical aspects of the disorder. The reader is also referred to Chapter 140 for additional details on disease mechanisms.
 PATHOLOGY AND PATHOPHYSIOLOGY
PATHOLOGY AND PATHOPHYSIOLOGY
A number of interrelated mechanisms contribute to the development and clinical course of ARDS. Inflammatory cytokines, oxygen radicals, activation of coagulation and complement, platelet and immune cell activation, generation of proteases, and abnormal fluid fluxes resulting in edema fluid generation and defective epithelial alveolar fluid clearance have all been hypothesized to play a role in the early stages.63–68 In addition, factors specific to apoptosis, edema fluid resolution, and fibrosis and repair, as well as the response to mechanical ventilation are likely to play a role in the pathophysiology of the later phases of ARDS.
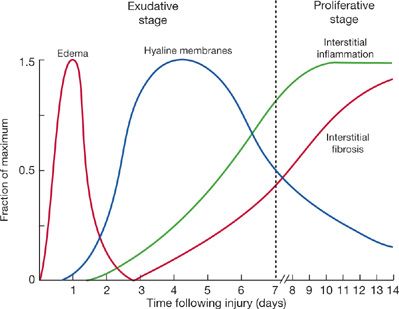
Pathologically and clinically, ARDS can be divided into early and late phases of lung injury (Fig. 141-1).69–72 In the early phase (first few hours or days), light microscopy shows interstitial and alveolar edema, capillary congestion, and intra-alveolar hemorrhage with minimal evidence of cellular injury. Electron microscopy reveals changes of endothelial cell swelling, widening of intercellular junctions, increased numbers of pinocytotic vesicles, and disruption and denudation of the basement membrane. Inflammatory cell infiltration of the lung interstitium may also be seen. Protein-rich pulmonary edema and its clinical effects are most pronounced in the early exudative phase. Hyaline membranes containing condensed fibrin and plasma proteins form over the next several days. Later, in the exudative phase, inflammatory cells become more numerous within the lung interstitium, and extensive necrosis of type I alveolar epithelial cells is present. Pathologists refer to this constellation of findings as diffuse alveolar damage (DAD).
Figure 141-1 Schematic representation showing time course of evolution of the acute respiratory distress syndrome (ARDS). The early or exudative phase is characterized by a pulmonary capillary leak with interstitial and alveolar edema and hemorrhage followed by hyaline membrane formation. Within as short a period of time as 7 to 10 days, a proliferative phase may appear with marked interstitial and alveolar inflammation and cellular proliferation, followed by fibrosis and disordered healing (see text for discussion). (Reproduced with permission from Katzenstein AA, Askin FB. Surgical Pathology of Non-Neoplastic Lung Diseases. 2nd ed. Philadelphia, PA: Saunders; 1990.)
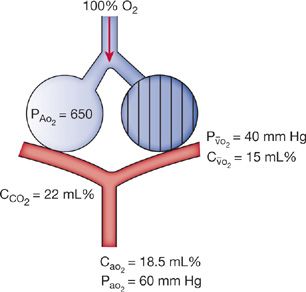
Pathophysiologically, in the exudative phase, alveolar edema and alveolar collapse, that is, atelectasis due to loss of normal surfactant-related stabilization of alveoli, interfere with oxygenation.73,74 Surfactant is washed out of alveoli and inactivated by the alveolar edema. The hypoxemia in ARDS is typically resistant to supplemental oxygen, reflecting an increased right-to-left shunt (Fig. 141-2). Continued perfusion of alveoli that lack ventilation because of alveolar edema results in ventilation–perfusion (![]() /
/![]() ) ratios of zero, thereby defining physiologic shunt. Furthermore, the effects of this type of shunt are exacerbated by shunt-like contributions from alveoli with very low ventilation–perfusion ratios.
) ratios of zero, thereby defining physiologic shunt. Furthermore, the effects of this type of shunt are exacerbated by shunt-like contributions from alveoli with very low ventilation–perfusion ratios.
Figure 141-2 Diagram of a two-compartment model of lung perfusion and ventilation demonstrating basis for failure of oxygenation in ARDS. When large portions of the lung are nonventilated due to alveolar collapse or flooding (hatched area), blood flow to these units with mixed venous PO2 (![]() ) of 40 mm Hg and content of 15 vol. percent is effectively “shunted” through the lungs without being resaturated. Thus, despite a high concentration of supplemental oxygen (100% in this example) and high alveolar PO2 in ventilated unit, these blood flows mix in accord with their oxygen contents, that is, the resulting left atrial blood has an oxygen content that is the weighted mean of the oxygen content of the shunted and nonshunted blood. In this example of a 50% shunt, the left atrial and systemic arteries have an arterial PO2 of 60 mm Hg. CaO2, arterial oxygen content; CCO2, capillaryoxygen content;
) of 40 mm Hg and content of 15 vol. percent is effectively “shunted” through the lungs without being resaturated. Thus, despite a high concentration of supplemental oxygen (100% in this example) and high alveolar PO2 in ventilated unit, these blood flows mix in accord with their oxygen contents, that is, the resulting left atrial blood has an oxygen content that is the weighted mean of the oxygen content of the shunted and nonshunted blood. In this example of a 50% shunt, the left atrial and systemic arteries have an arterial PO2 of 60 mm Hg. CaO2, arterial oxygen content; CCO2, capillaryoxygen content; ![]() , mixed venous oxygen content; PA, alveolar pressure; PaO2, arterial PO2;
, mixed venous oxygen content; PA, alveolar pressure; PaO2, arterial PO2; ![]() , partial pressure of oxygen in the mixed venous blood. (Reproduced with permission from Christie JD, Lanken PN. Acute lung injury and the acute respiratory distress syndrome. In: Hall JB, Schmidt GA, Wood LDH, eds. Principles of Critical Care. 3rd ed. New York, NY: McGraw-Hill; 2005.)
, partial pressure of oxygen in the mixed venous blood. (Reproduced with permission from Christie JD, Lanken PN. Acute lung injury and the acute respiratory distress syndrome. In: Hall JB, Schmidt GA, Wood LDH, eds. Principles of Critical Care. 3rd ed. New York, NY: McGraw-Hill; 2005.)
Disordered healing and proliferation of fibrous tissue dominate the late phase of ARDS or persistent ARDS, that is, the proliferative or fibroproliferative phase. Type II alveolar cells, fibroblasts, and myofibroblasts proliferate in this phase, which can occur as early as 7 to 10 days after initial injury. The late phase of ARDS is characterized by an increased dead-space fraction, high minute ventilation requirement, pulmonary hypertension, and further reduction in lung compliance.75,76
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
The development of ARDS usually follows a rapid course, occurring most often within 12 to 72 hours of the predisposing event.38 At its onset, patients with ARDS often become anxious, agitated, and dyspneic. Inflammatory changes in the lung decrease lung compliance, which, in turn, leads to an increased work of breathing, small tidal volumes, and tachypnea. If breathing ambient air or low-flow supplementary oxygen, patients with ARDS typically have initial arterial blood gas results showing a PaO2 less than 50 to 55 mm Hg and pulse oximetry recordings of less than 85% arterial O2 saturation. The hallmark of ARDS is hypoxemia that is resistant to oxygen therapy because of the large right-to-left shunt (Fig. 141-2). Initially, patients may be able to compensate by hyperventilating, thereby maintaining an acceptable PaO2 with an acute respiratory alkalosis. Typically, patients deteriorate over several hours, requiring endotracheal intubation and mechanical ventilation.
 DIFFERENTIAL DIAGNOSIS
DIFFERENTIAL DIAGNOSIS
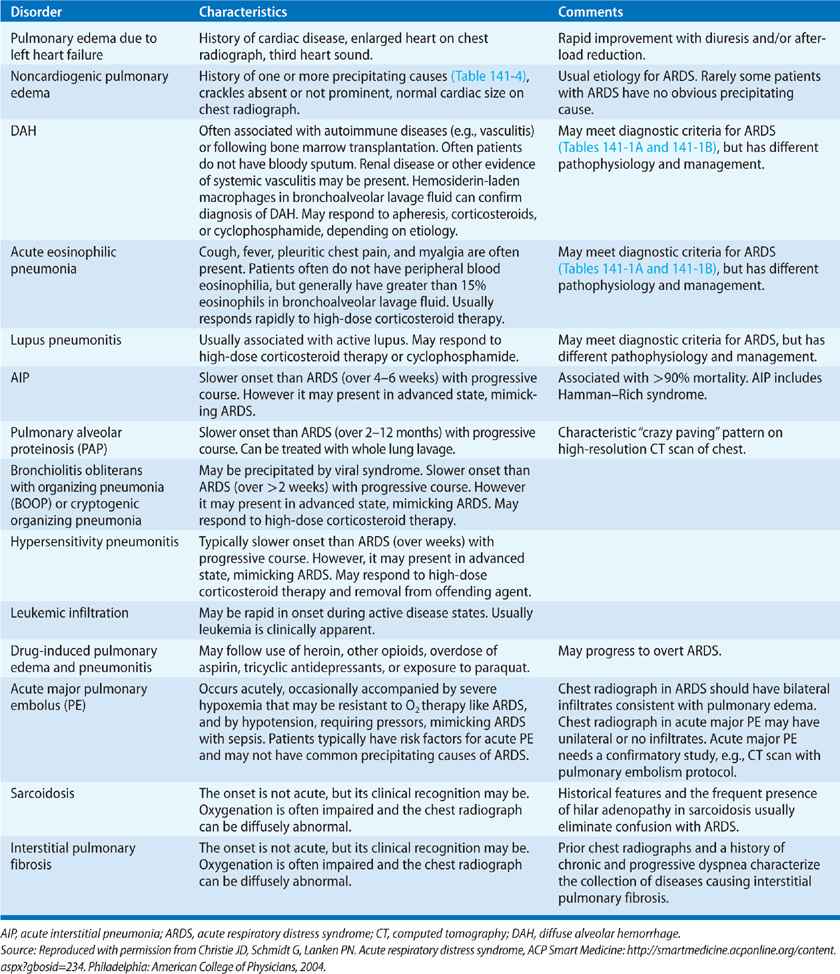
The differential diagnoses for acute hypoxemic respiratory failure, in general (Table 141-5), and for ARDS, in particular (Table 141-6), are extensive.77 Identifying the specific etiology of the diffuse infiltrates in ARDS is important because several causes, for example, acute eosinophilic pneumonia or diffuse alveolar hemorrhage, have specific therapies. Table 141-6 lists the major clinical and diagnostic characteristics of these disorders.
ARDS, acute respiratory distress syndrome.
Source: Reproduced with permission from Christie JD, Schmidt G, Lanken PN. Acute respiratory distress syndrome, ACP Smart Medicine: http://smartmedicine.acponline.org/content.aspx?gbosid=234. Philadelphia: American College of Physicians, 2004.
The setting in which respiratory failure occurs usually provides important diagnostic information. ARDS commonly arise following development of a typical predisposing factor (Table 141-4). Sepsis, pneumonia, trauma, transfusion of blood products, and gastric aspiration account for the majority of cases.38 When an inciting event is obvious and diagnostic criteria (Tables 141-1A and 141-1B) are met, establishment of a clinical diagnosis of ARDS is not difficult. Under such circumstances, management can be instituted immediately. However, in the absence of a clear predisposing event, or when conflicting or ambiguous information exists, the other causes listed in Table 141-6 should be considered, and relevant clues from the history and physical examination sought. For example, cardiogenic edema is most often accompanied by systolic left ventricular or valvular dysfunction, and the appropriate history and physical findings (e.g., a heart murmur or ventricular gallop) are often present. Electrocardiographic and laboratory-based evidence (e.g., serum troponin I levels) of cardiac ischemia suggest cardiogenic edema as a likely cause. Additional important tests that help to differentiate ARDS from other causes of acute hypoxemic respiratory failure are discussed subsequently.
 APPROACH TO CLINICAL DIAGNOSIS
APPROACH TO CLINICAL DIAGNOSIS
A number of diagnostic methods are extremely valuable in evaluating suspected ARDS. Each is briefly described.
Chest Radiograph
The chest radiograph is a simple and widely available test used to assess patients with acute hypoxic respiratory failure. In cases of established ARDS, the chest radiograph typically demonstrates findings of diffuse, bilateral alveolar infiltrates consistent with pulmonary edema (Fig. 141-3). However, especially early in the course of the disorder, the infiltrates associated with ARDS may be variable: mild or dense, interstitial or alveolar, patchy or confluent.7 In addition, the radiographic infiltrates may not correlate well with the degree of hypoxemia. For example, a patient with early ARDS may have profound hypoxemia in the setting of patchy asymmetrical infiltrates that may be interpreted as pneumonia or segmental atelectasis.
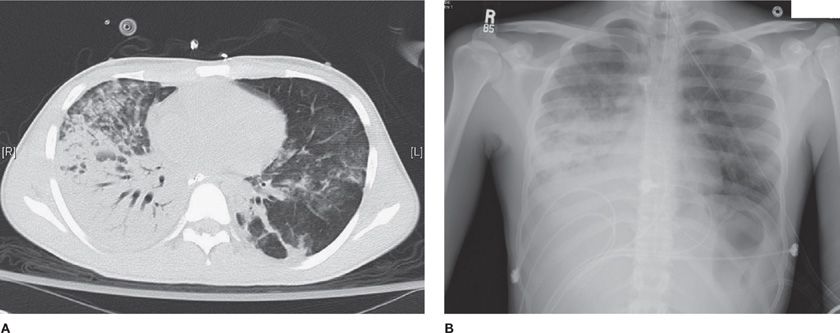
Figure 141-3 Chest CT and plain radiograph in ARDS. A. Chest CT scan reveals asymmetric lung injury, with dense consolidation at the right base, patchy alveolar infiltrates in the right anterior lung field, and patchy ground-glass infiltrates throughout the right lung. B. Chest radiograph obtained concurrently with chest CT scan shown in panel (A). Dense infiltrates at right base, patchy infiltrates in the right upper lung zone, and more subtle infiltrates in the left lung are demonstrated. The panels illustrate the subtle findings of lung injury that are more apparent on the CT scan than on the chest radiograph.
Routine chest radiographs cannot reliably distinguish hydrostatic edema, that is, cardiogenic edema, from ARDS. Nonetheless, several criteria suggest cardiogenic edema: increased heart size, increased width of the vascular pedicle, vascular redistribution toward upper lobes, the presence of septal lines, or a perihilar (bat’s wing) distribution of the edema.78 Lack of these findings, in conjunction with patchy peripheral infiltrates that extend to the lateral lung margins, suggests ARDS. In the proper clinical setting, despite a variable radiographic appearance, the presence of bilateral infiltrates and moderate or severe hypoxemia (PaO2/FIO2 ≤300 mm Hg) should raise the possibility of ARDS.
Laboratory Studies
Although no laboratory test is specific for the diagnosis of ARDS, arterial blood gas analysis is essential for confirming the diagnosis.9 PaO2/FIO2 is markedly abnormal in patients with ARDS (Tables 141-1A and 141-1B). In addition to the profound oxygen therapy–resistant hypoxemia that is the hallmark of ARDS, acute respiratory alkalosis may also occur in the early stage. If a patient with ARDS then develops respiratory muscle fatigue, hypercapnia results. In late-stage ARDS, patients typically have increased minute ventilation requirements due to an increasing dead-space fraction, despite possible improvement in oxygen exchange.
In addition to arterial blood gas measurements, several other laboratory studies may be helpful in investigating other causes of respiratory failure and evaluating additional aspects of critical illness associated with ARDS. For example, cardiac enzymes (creatine phosphokinase and troponins) are useful for evaluating the presence of myocardial infarction or cardiac ischemia in patients at risk because of increased age or other factors. The results should be interpreted in conjunction with electrocardiographic findings, since elevations in cardiac enzymes, especially troponins, have been reported in patients with sepsis or septic shock in the absence of coronary artery disease.79,80
Another cardiac-related laboratory test that may be useful in this clinical context is plasma brain natriuretic peptide (BNP), which is secreted by the cardiac ventricles, and, to a lesser extent, the atria. BNP measurements are often utilized in the evaluation of acute shortness of breath in patients presenting to an emergency department.81 In this group, a BNP greater than 500 pg/mL indicates that CHF is likely with a positive predictive value greater than 90%. In the same group, a BNP less than 100 pg/mL suggests that congestive heart is unlikely with a negative predictive value greater than 90%. However, interpretation of an elevated BNP in patients who are critically ill is problematic. Reports indicate that BNP increases with renal failure, and that elevations of BNP greater than 500 pg/mL may occur in patients with sepsis and normal left ventricular function.82 Nonetheless, one can reasonably exclude a cardiac cause for acute pulmonary edema in patients in the intensive care unit if BNP is less than 100 pg/mL.
Echocardiography
Echocardiography is a useful noninvasive method to evaluate potential cardiac causes of acute hypoxemic respiratory failure.83 Cardiogenic pulmonary edema is suggested by echocardiographic findings of mitral valve stenosis or regurgitation, left ventricular dilatation and systolic dysfunction, or regional left ventricular wall motion abnormalities. Although these findings do not rule out coexisting lung injury, they are helpful in the initial evaluation and management, even in the presence of ARDS.
Invasive Hemodynamic Monitoring
Although pulmonary artery catheterization has been performed often in patients with pulmonary edema, the benefits of the procedure are controversial and the topic of recent investigations (see Chapter 147, Hemodynamic and Respiratory Monitoring in Acute Respiratory Failure).11,84 Several studies have demonstrated that physician interpretation of data obtained from PACs is inconsistent and often erroneous.85–87 Previous definitions of ARDS have suggested the measurement of PAOP to distinguish ARDS from cardiogenic pulmonary edema3; however, the recently accepted Berlin definition of ARDS eliminates such suggestions, recognizing the problems inherent in use of PACs.9 Studies have demonstrated that many patients who originally met AECC criteria for ARDS (i.e., had a PAOP ≤18 mm Hg) often have subsequent measurements with a PAOP >18 mm Hg.12,88 Furthermore, the Berlin definition recognizes the possibility of concomitant ARDS and cardiogenic edema.
Results of the ARDSNet Fluid and Catheter Treatment Trial (FACCT) support the removal of PAOP criteria from the definition of ARDS, acknowledging that a threshold of 18 mm Hg is arbitrary.11 FACTT was a large, randomized clinical trial that used a two-by-two factorial design to test a fluid-conservative management strategy against a fluid-liberal management strategy in ARDS. The study also assessed safety and efficacy of a central venous catheter (CVC) or PAC to guide fluid management. In FACTT, 29% of 513 patients enrolled in the PAC arm of the trial were found to have a PAOP greater than 18 mm Hg at the time of initial measurement (following passage of the catheter shortly after enrollment and randomization). Before enrollment, FACTT investigators believed that these patients lacked a primary cardiogenic cause for their pulmonary edema. Approximately one-half of the patients had PAOPs of 19 or 20 mm Hg. Since the vast majority (97%) of this group had a normal cardiac index (≥2.5 L/m2/min) and a mortality similar to other subjects in FACTT, the elevated PAOP (>18 mm Hg) likely reflected intravascular volume loading, rather than cardiogenic pulmonary edema.
Bronchoalveolar Lavage
Bronchoscopy with bronchoalveolar lavage (BAL) is an important tool in the evaluation of patients who have ARDS of unclear origin. In general, BAL can be performed safely in patients with ARDS, except in those with a very low PaO2 or those requiring high levels of PEEP. The principal reason for performing bronchoscopy in ARDS is to rule in or rule out acute processes that may have specific therapies.
For example, acute eosinophilic pneumonia is a rare disorder characterized by diffuse eosinophilic infiltrates in the lungs (Table 141-6).89 When the precipitating cause for ARDS is uncertain, performance of BAL and measurement of the percent of eosinophil count in the lavage fluid is helpful in establishing a diagnosis of this corticosteroid-responsive disorder.
Likewise, BAL may be diagnostic for diffuse alveolar hemorrhage (see Chapter 68, Alveolar Hemorrhage Syndromes).90 In this case, the bronchoscopy may or may not reveal fresh blood in the trachea and major bronchi. However, BAL generally demonstrates blood-tinged fluid, which contains red blood cells and hemosiderin-laden macrophages. Diffuse alveolar hemorrhage may occur following bone marrow transplantation or as a result of rheumatologic or other immunologic disorders, including Goodpasture syndrome, granulomatosis with polyangiitis (formerly known as Wegener granulomatosis), systemic lupus erythematosus, or antiphospholipid antibody syndrome.
Lung Biopsy
Routine lung biopsy is not recommended in ARDS; however, lung biopsy should be considered if alternative etiologies of respiratory failure cannot be adequately excluded. While many patients with ARDS are not sufficiently stable to undergo a lung biopsy, the safety of a surgical lung biopsy has been demonstrated in a highly selective group of patients with hypoxic respiratory failure.91,92 In general, lung biopsy should be reserved for a highly selective group of patients where alternative diagnoses are possible and would significantly change management and prognosis.
APPROACH TO TREATMENT
The general approach to treatment of ARDS includes addressing precipitating causes and other concurrent clinical issues, ensuring adequate oxygenation, careful implementation of a lung-protective ventilator strategy, prudent fluid and hemodynamic management, special patient positioning coupled with the so-called “lung recruitment” maneuvers, and a multitude of other measures, including pharmacologic considerations. Each is discussed subsequently.
 GOALS OF MANAGEMENT
GOALS OF MANAGEMENT
Management of patients with ARDS can be complicated and challenging because clinicians are often faced with simultaneous failure of both respiratory and nonrespiratory organ systems (Table 141-7). Unfortunately, only a limited set of controlled clinical trials are available to support an evidence-based approach. For example, even large, multicenter, randomized clinical trials, such as those done by ARDSNet, are limited in the number of variables that can be tested. As a result, patient management rests on a combination of relevant evidence-based medicine, extrapolations from basic and clinical research, and experience-based approaches.
 DIAGNOSIS AND TREATMENT OF PRECIPITATING CAUSES AND OTHER COMORBIDITIES
DIAGNOSIS AND TREATMENT OF PRECIPITATING CAUSES AND OTHER COMORBIDITIES
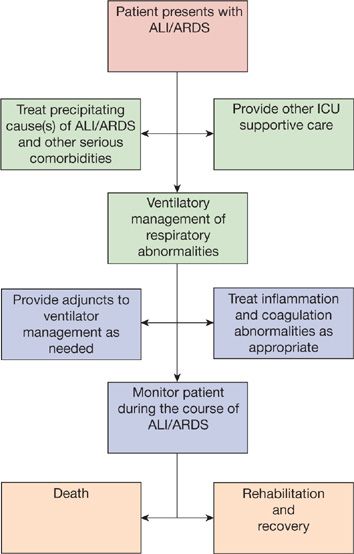
The first step in the therapy of ARDS is identification and treatment of the precipitating cause(s) and any other life-threatening medical or surgical issues (Fig. 141-4).
Figure 141-4 Summary of treatment approach to ARDS. Note that, “Treat inflammation and coagulation abnormalities as appropriate,” is currently limited. In the ARDSNet “LaSRS” clinical trial, physiologic improvement, but no mortality benefit, was found with high-dose corticosteroid therapy for persistent (late phase) ARDS (see text for details). (Reproduced with permission from Christie JD, Lanken PN. Acute lung injury and the acute respiratory distress syndrome. In: Hall JB, Schmidt GA, Wood LDH, eds. Principles of Critical Care. 3rd ed. New York, NY: McGraw-Hill; 2004.)
Since ARDS is a syndrome based on nonspecific radiographic and physiologic criteria, establishing a diagnosis of ARDS is not equivalent to diagnosing the precipitating cause. The fact that early identification and treatment directed at the inciting cause(s) of ARDS are imperative for the resolution of lung injury and respiratory failure cannot be overemphasized. Treatable inciting causes of ARDS include a variety of infectious and noninfectious disorders (Table 141-8).
Source: Reproduced with permission from Christie JD, Lanken PN. Acute lung injury and the acute respiratory distress syndrome. In: Hall JB, Schmidt GA, Wood LDH, eds. Principles of Critical Care, 3rd ed. New York, NY: McGraw-Hill; 2004.
 MANAGEMENT OF RESPIRATORY FAILURE
MANAGEMENT OF RESPIRATORY FAILURE
Management of respiratory failure in ARDS rests on assurance of adequate oxygenation and carefully crafted ventilatory strategies, as outlined in the following sections.
Maintaining Adequate Oxygenation
As noted, the pathophysiologic hallmark of ARDS is hypoxemia that is resistant to oxygen therapy. Maintaining adequate arterial oxygenation is the primary goal of both traditional and newer (“lung-protective”) approaches to assisted ventilation.
As expected with shunt physiology, administration of supplemental oxygen provided by high-flow oxygenation systems, for example, a nonrebreather face mask, is generally ineffective in reversing the oxygenation deficit. Exceptions to this rule are some patients with mild or transient cases of ARDS that are otherwise uncomplicated by other organ system failures.
Upon intubation, most patients with ARDS require high levels of FIO2 to maintain oxygenation; however, high concentrations of oxygen may potentially be toxic. While not studied adequately in patients with ARDS, studies of animals and healthy humans have demonstrated the toxic effects of oxygen therapy within hours of its initiation.93,94 Given the theoretical concern for oxygen toxicity, PEEP is employed to improve oxygenation by reducing physiologic shunt. When utilized in sufficient amounts, PEEP generally results in some degree of correction of the hypoxemia in patients with ARDS, thereby allowing FIO2 to be lowered from high, potentially toxic concentrations. Although PEEP is usually used in conjunction with mechanical ventilation, in selected cases of mild ARDS it may be effective when applied by means of a continuous positive airway pressure (CPAP) mask or as the lower level of bilevel noninvasive ventilation. The effect of PEEP-induced improvement in arterial oxygenation is attributed predominantly to recruitment of collapsed alveoli. However, application of PEEP may also mediate a redistribution of alveolar fluid into the interstitium and decrease the absolute magnitude of shunt by reducing cardiac output.
Acutely ill patients in intensive care units typically receive assisted ventilation via an endotracheal tube. In selected non-ARDS disorders, for example, COPD or acute cardiogenic pulmonary edema, noninvasive ventilation has been shown to be as effective as invasive ventilation.95,96 Although the routine use of noninvasive ventilation for patients with ARDS lacks compelling evidence, the data are limited. In a small randomized trial of 40 patients with mild ARDS (formally referred to as ALI), patients receiving noninvasive ventilation were less likely to require intubation than those receiving supplemental oxygen alone; however, the study was small and not fully blinded.97 One reason for limited study of noninvasive ventilation in ARDS is the high incidence of concomitant, nonrespiratory organ failure (e.g., multisystem organ failure due to septic shock). Except for select subgroups, for example, immunosuppressed patients with hypercapnic respiratory failure who are hemodynamically stable, use of noninvasive ventilation for patients with ARDS generally should be avoided.
Lung-Protective Mechanical Ventilation
As described in Chapter 140, over the past 30 years investigators have convincingly shown that large tidal volumes delivered during mechanical ventilation can injure lungs of normal animals, producing a pathologic pattern resembling ARDS in humans.98,99
In animal models of ALI, use of large tidal volume ventilation has been found to augment pre-existing injury. In addition, repetitive opening and closing of alveoli during inspiration and expiration induces ALI in normal animals.100,101 The injury can be prevented by application of sufficient PEEP. Finally, overexpansion of alveoli in normal lungs of sheep induces multiorgan failure,102 with studies in other species showing that lung overexpansion results in systemic release of proinflammatory cytokines—providing a likely mechanism for these remote deleterious effects.103–105 These observations support the concept that the lung is an “engine of inflammation.”
Concurrent with the previously described observations, clinical investigators studying patients with ARDS using computed tomography observed that, in contrast to the typical diffuse-appearing pattern noted on plain chest radiographs, the pattern of consolidation, atelectasis, and normal alveoli is actually heterogeneous (Fig. 141-3).106 The key physiologic implication of these observations is that a ventilator-delivered tidal volume is preferentially distributed to the open alveoli, which represent only a small fraction of the entire lung.107 In referring to this lung fraction as “the baby lung” investigators emphasized the potential danger of delivering traditional tidal volumes of 10 to 15 mL/kg actual body weight and the associated risk for alveolar overexpansion and lung injury. Notably, tidal volumes of 10 to 15 mL/kg actual body weight (equivalent to approximately 12 to greater than 15 mL/kg predicted body weight) were used originally in critically ill patients with ARDS as a complementary strategy to PEEP in recruiting atelectatic alveoli.
These productive lines of basic and clinical research strongly support the hypothesis that mechanical ventilation using limited tidal volumes should be less injurious to the lungs of patients with ARDS and should result in better outcomes (i.e., decreased mortality) compared with use of traditional, large tidal volume ventilation. Several randomized controlled trials have been conducted to address the role of low tidal volume ventilation in ARDS, including the landmark ARDSNet trial, ARMA, discussed in detail below.4,108 Recent meta-analyses of multiple trials support a low tidal volume ventilation approach to reduce mortality in ARDS.109,110
In summary, the goals of lung-protective ventilation are to avoid injury due to overexpansion of alveoli during inspiration (so-called “volu-trauma”) and injury due to repetitive opening and closing of alveoli during inspiration and expiration (so-called “atelecta-trauma”) (Fig. 141-5). The injurious effects of mechanical ventilation on the lung have been referred to as ventilator-induced lung injury or “VILI.” The term “bio-trauma” encompasses the direct lung injury and the concomitant release of inflammatory cytokines that produce remote cell death or organ injury.111 Clinical strategies underlying contemporary applications of mechanical ventilation in treatment of ARDS are described subsequently.
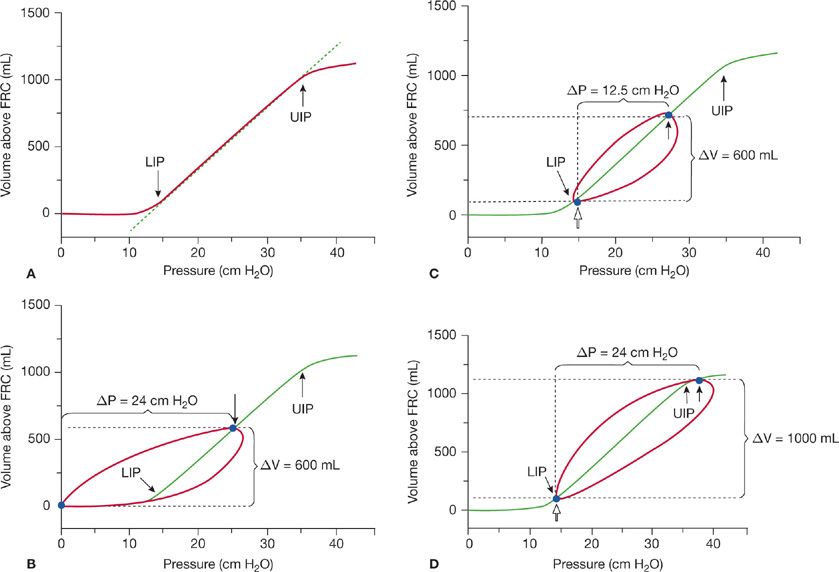
Figure 141-5 A. Schematic inspiratory static pressure–volume (P–V) curve of the respiratory system (lung and chest wall combined) in ARDS. Lower inflection point (LIP) is approximately 14 cm H2O, and upper inflection point (UIP) is approximately 35 cm H2O. Abscissa is respiratory system recoil pressure; ordinate is lung volume above functional residual capacity (FRC). B. Same static P–V curve as (A) plus dynamic P–V curve of 600 mL tidal volume starting below the LIP (PEEP = 0). This tidal volume results in a plateau pressure (closed arrow) below the UIP (24 cm H2O). Static compliance (Cstat = ΔV/ΔP = 600 mL/24 cm H2O) is 25 mL/cm H2O. C. PEEP of 15 cm H2O has moved the starting point for the 600 mL tidal volume up the static P–V curve to a new FRC (open arrow), which is at the LIP. The tidal volume results in a plateau pressure of 27.5 cm H2O (closed arrow), which is well below the UIP. Cstat (ΔV/ΔP = 600 mL/12.5 cm H2O) is increased to 48 mL/cm H2O. D. Dynamic P–V curve of a 1000 mL tidal volume, starting at 14 cm H2O PEEP, results in a plateau pressure of 37.5 cm H2O (closed arrow). Despite an increase in Cstat (ΔV/ΔP = 1000 mL/24 cm H2O = 41.5 mL/cm H2O), compared with Cstat derived from the 600 mL tidal volume in (B), the plateau pressure associated with the 1000 mL tidal volume exceeds the UIP. Delivery of an inflation volume that results in a plateau pressure exceeding the UIP implies alveolar overdistension and is believed to put the lung at risk for ventilator-induced lung injury (see text). (Reproduced with permission from Lanken PN. Acute respiratory distress syndrome. In: Lanken PN, Hanson CW III, Manaker S, eds. The Intensive Care Unit Manual. Philadelphia, PA: Saunders; 2001.)
ARDSNet Ventilator Strategies: Low Versus Traditional Tidal Volumes Based on the aforementioned considerations, the ARDSNet conducted a randomized trial (ARMA) in the mid-to-late 1990s to test the hypothesis that low tidal volume ventilation, combined with limited end-inspiratory (plateau) pressure, would lower mortality and ventilator days among survivors of ARDS compared with use of traditional tidal volumes.4 The trial included 861 subjects. The low tidal volume arm consisted of a tidal volume of 6 mL/kg predicted body weight, as long as the end-inspiratory pressure (Pplat) was ≤30 cm H2O; if Pplat exceeded 30 cm H2O, the tidal volume could be decreased to as low as 4 mL/kg. The traditional tidal volume arm used a tidal volume of 12 mL/kg predicted body weight, as long as the Pplat remained <50 cm H2O. Both arms included explicit goals and protocols as the bases for ventilator adjustments and determination of the time and means of weaning (Table 141-9).