aRating scale: 1, 2, 3 usually not appropriate; 4, 5, 6 may be appropriate; 7, 8, 9 usually appropriate.
Adapted from American College of Radiology ACR Appropriateness Criteria®, 2013.9
US, ultrasound; MRV, magnetic resonance venography; CTV, computed tomographic venography; DVT, deep vein thrombosis.
TABLE 19.2 American College of Cardiology Foundation (ACCF) Appropriate Use Criteria for Lower Extremity Venous Duplex Ultrasound
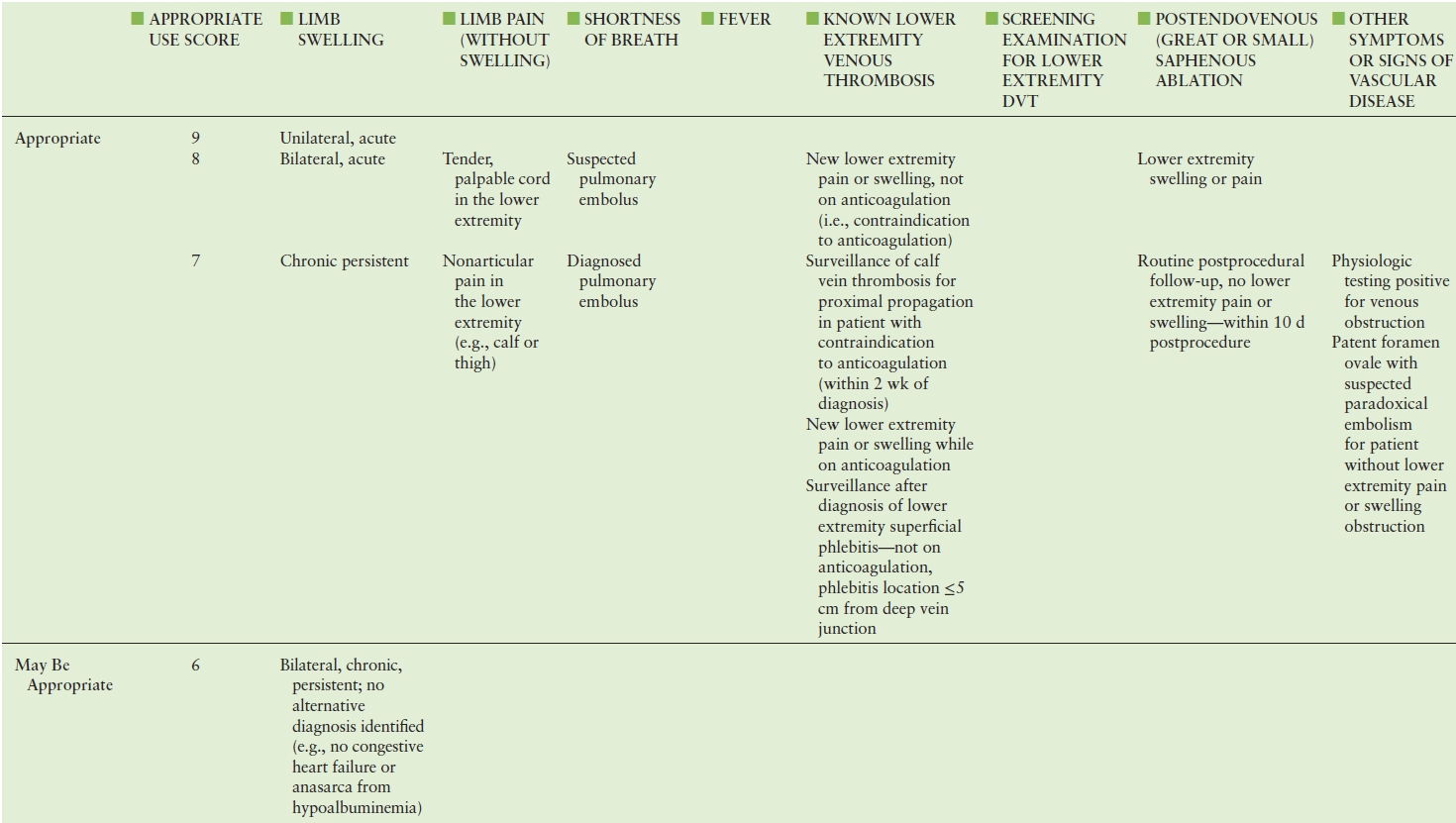
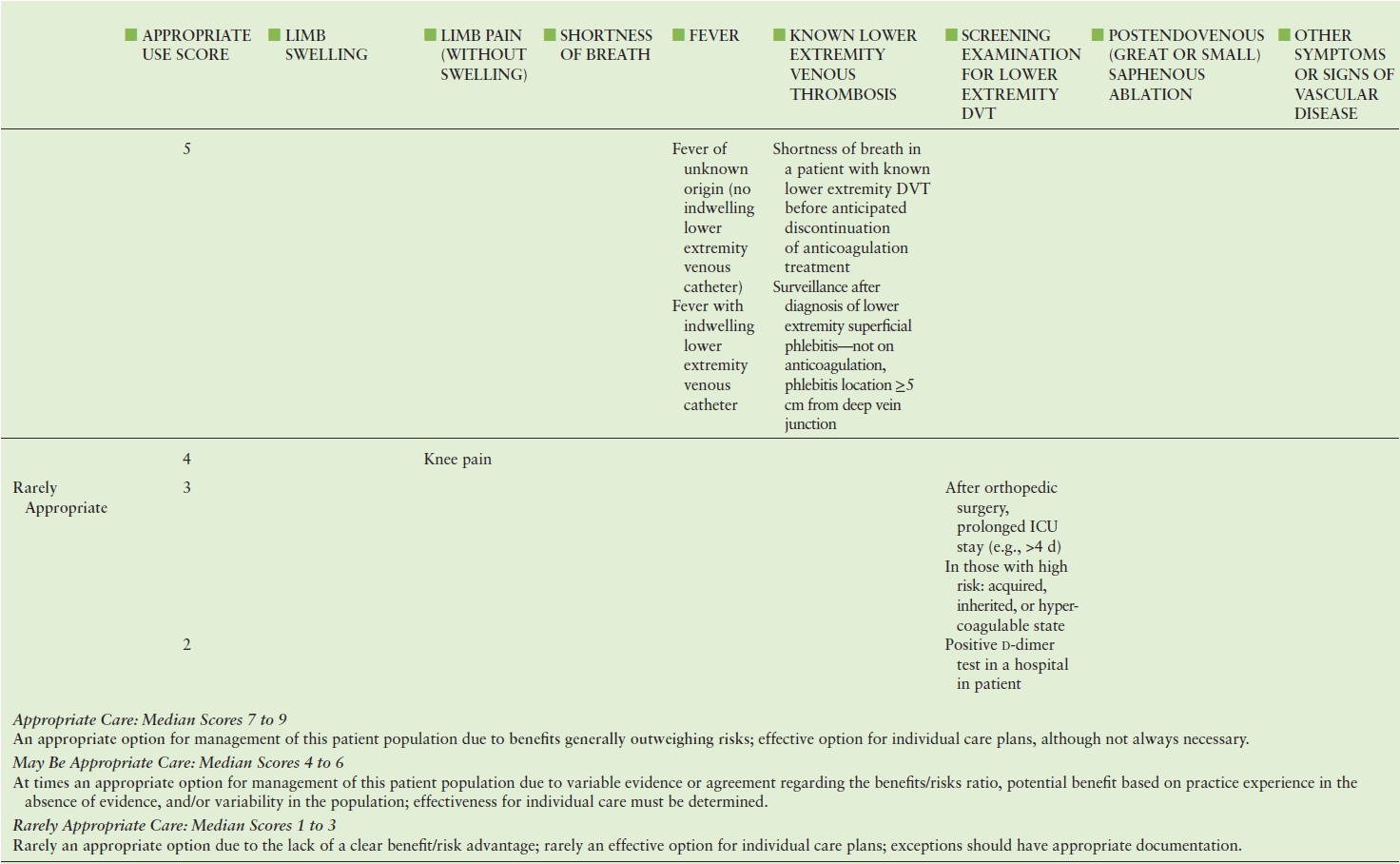
Adapted from: American College of Cardiology Foundation Appropriate Use Criteria Task Force; American College of Radiology; American Institute of Ultrasound in Medicine. ACCF/ACR/AIUM/ASE/IAC/SCAI/SCVS/SIR/SVM/SVS/SVU 2013 appropriate use criteria for peripheral vascular ultrasound and physiological testing. Part II: Testing for venous disease and evaluation of hemodialysis access. Vasc Med 2013;18(4): 215–231. doi: 10.1177/1358863X13497637. PMID: 23897935.
EPIDEMIOLOGY OF VENOUS THROMBOEMBOLISM
DVT and pulmonary embolism (PE), jointly termed “venous thromboembolism” (VTE), represent a major clinical problem. Prevalence estimates suggest that there are over 900,000 VTE cases annually in the United States, with as many as 300,000 PE deaths each year.13 After heart disease and stroke, VTE is the third most common vascular disease. To put the magnitude of the problem in perspective, it has been pointed out that deaths from PE are five times more common than deaths from breast cancer, motor vehicle accidents, and AIDS combined.14 Data used by the American College of Chest Physicians (ACCP) for VTE risk estimation have suggested that 31% of hospitalized patients are at risk for VTE, which represents approximately 12 million patients per year.15
In addition to the acute risks associated with DVT, many patients subsequently develop symptoms of postthrombotic syndrome (PTS), with pain and limb swelling that can be debilitating (see Chapter 21). These symptoms can develop months or years after the acute episode of thrombosis.16,17 PTS can substantially impact quality of life. The overall incidence of PTS is estimated to be as high as 30%, although the incidence of symptoms may be higher for some subgroups of DVT patients, in particular those with iliofemoral venous thrombosis.18
The significance of VTE as a public health problem prompted the US Surgeon General to issue a “Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism” in 2008.19 This initiative urged greater understanding of the risk factors and triggering events for developing DVT and PE, better recognition of the symptoms, and implementation of steps to prevent and treat these serious conditions. VTE patient safety measures were developed as a result of the National Consensus Standards for the Prevention and Care of Deep Vein Thrombosis project between the Joint Commission and the National Quality Forum (NQF). Six VTE measures were endorsed by the NQF in May 2008, and these aligned with priorities of the Centers for Medicare and Medicaid Services.20 Joint Commission Hospital National Patient Safety Goals emphasize the need for appropriate prophylactic measures.21
RISK FACTORS
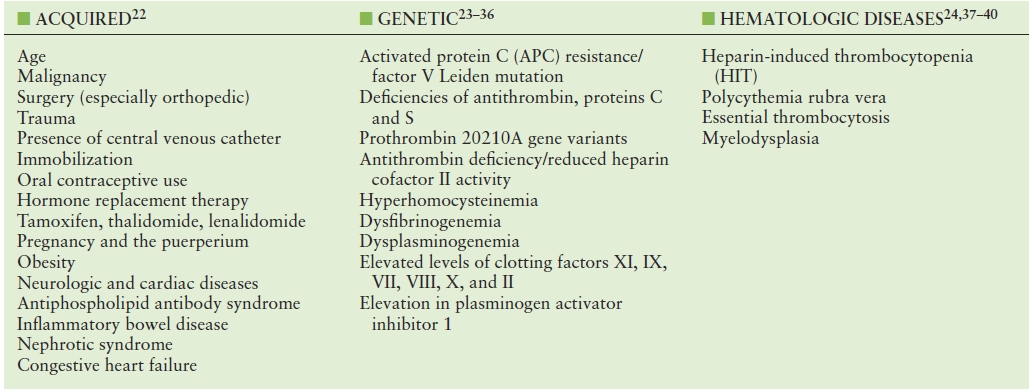
A number of VTE risk factors have been identified, and new factors are currently being investigated. Individuals with an inherited blood clotting disorder or who experience a triggering event such as hospitalization, surgery, or long periods of immobility are more likely to develop DVT or PE. The risk for DVT and PE increases with age, especially after age 50. Women who are pregnant or take hormones (for birth control or menopausal therapy) are at increased risk. Predisposing conditions are summarized in Table 19.3.41–59 Screening for a hypercoagulable state should be considered for atypical presentations of venous thrombosis,60 including thrombosis in unusual locations (i.e., mesenteric veins or portal vein), idiopathic venous thrombosis, recurrent venous thrombosis, and superficial venous thrombosis (SVT) in a nonvaricose great saphenous vein.
TABLE 19.3 Risk Factors Associated with Venous Thromboembolism (VTE)
CLINICAL DIAGNOSIS OF DVT
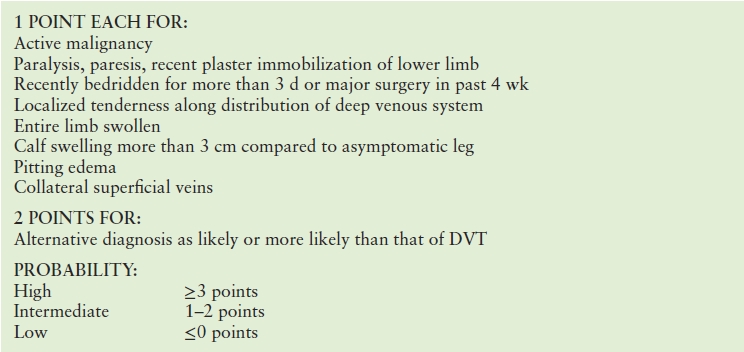
Anticoagulation for proximal DVT prevents thromboembolic complications in most patients, and it effectively prevents propagation of distal limb thrombi,61 but anticoagulation is inconvenient and costly and can be associated with serious bleeding complications, so indiscriminant use of anticoagulant therapy is to be avoided. Thus, it is necessary to accurately make the diagnosis of DVT. The clinical diagnosis of DVT is notoriously inaccurate, lacking both sensitivity and specificity.62 The optimal strategies for clinically assessing DVT risk (pretest probability) consider a combination of demographic and clinical factors. Several risk-scoring algorithms have been developed, including that from Wells et al (Table 19.4).63 DVT risk estimates based on data from ambulatory and hospitalized populations may differ. Some have suggested that DVT prevalence may be higher in primary care settings than might be expected using the Wells calculations.64
TABLE 19.4 Wells Score for Estimating Probability of Deep Vein Thrombosis
d-Dimer
The d-dimer antigen is a marker of fibrin degradation from the action of thrombin, factor XIIIa, and plasmin on thrombus. Fibrin degradation products are created, exposing the d-dimer antigen. d-dimer antigen can exist on fibrin degradation products derived either from soluble fibrin or from fibrin clot that has been degraded by plasmin. d-dimers are unique fibrin degradation products, as they are produced only from the lysis of cross-linked fibrin (factor XIII cross-links the E-element to two D-elements).
The d-dimer concentration will be elevated in the presence of DVT, though an elevated d-dimer is nonspecific. In other words, a positive d-dimer result can indicate thrombosis, but it has other potential causes. This means a normal d-dimer measurement can be helpful for the exclusion of acute VTE, but a positive result does not confirm a DVT diagnosis, as d-dimer is a marker of activation of the coagulation system in other settings.65 The d-dimer level can be high due to liver disease, inflammation, malignancy, trauma, pregnancy, and recent surgery, as well as advanced age. False negatives with d-dimer testing can be encountered if a blood sample is obtained early in the process of thrombus formation, with limited thrombosis (e.g., limited to calf veins), or if testing is delayed for several days. Additionally, use of anticoagulant therapy can interfere with the test. Thus, the principal effectiveness of d-dimer testing is to exclude the diagnosis VTE when its probability is low, based on clinical criteria.66
Use of Combined Prediction Rules for DVT Diagnosis
Bayes’ theorem is the statistical principle which states that the likelihood of a test yielding a true positive is highest if the test is applied to a population with a high prevalence of the condition that is the subject of the test. The diagnostic accuracy of testing for DVT improves when clinical probability is estimated before diagnostic tests are performd.67 Due to the poor specificity of d-dimer testing for DVT, this laboratory test should be used as one element of an integrated diagnostic strategy that includes clinical probability assessment and imaging.68
In a systematic review of 14 studies involving more than 8,000 outpatients, the value of clinical prediction rules for the diagnosis of DVT, either with or without d-dimer, was evaluated.69 The studies included all had sufficient information to allow the calculation of the prevalence of DVT for at least one of the three clinical probability estimates (low, moderate, or high) and had patient follow-up for a minimum of 3 months. The prevalence of DVT in the low, moderate, and high clinical probability groups was 5.0%, 17%, and 53%, respectively. The overall prevalence of DVT was 19%. Pooling the studies, the sensitivity and specificity of d-dimer testing in the low-probability group were 88% and 72%; in the moderate-probability group, 90% and 58%; and in the high-probability group, 92% and 45%. The likelihood ratio for a normal result on a highly sensitive d-dimer assay among patients with (1) low clinical suspicion was 0.10, (2) moderate clinical suspicion was 0.05, and (3) high clinical suspicion was 0.07. Thus, it was very uncommon to have a normal d-dimer result in settings where DVT was likely. Patients with low clinical probability on the predictive rules have a prevalence of DVT of less than 5%.
Another review by Fancher et al analyzed 12 studies with more than 5000 outpatients tested with a rapid d-dimer assay after categorization into low, intermediate, or high clinical probability of DVT on the basis of clinical criteria. Using a less sensitive d-dimer assay, the 3-month incidence of VTE was 0.5% among patients with a low clinical probability of DVT and a normal d-dimer concentration. When a highly sensitive d-dimer assay was used, the 3-month incidence of VTE was 0.4% among outpatients with low or moderate clinical probability of DVT and a normal d-dimer concentration.70
To summarize, in low-probability patients with negative d-dimer results, a diagnosis of DVT can be excluded without additional diagnostic testing (e.g., venous duplex scan), but in patients for whom there is a high clinical suspicion for DVT, d-dimer results should not affect clinical decisions.
VASCULAR LABORATORY TESTS FOR DVT
Plethysmography
Plethysmographic techniques are indirect tests that evaluate venous hemodynamics by assessing the changes in limb volume that occur as the venous capacitance vessels fill or empty. These techniques include impedance plethysmography (IPG), air plethysmography (AP),71 mercury strain gauge plethysmography (SGP),72,73 photoplethysmography (PPG),74–76 and foot volumetry. The limb increases in volume when a thigh cuff is inflated to a pressure below arterial but above the venous pressure. Normally, limb volume rapidly returns toward baseline with release of the thigh cuff. In most cases, half of the venous emptying occurs in the first 2 seconds after cuff release.
IPG was an early vascular laboratory standard for diagnosis of DVT associated with hemodynamically significant venous outflow obstruction.77–80 The impedance plethysmograph measured changes in electrical impedance in an extremity after inflation and release of a proximal venous tourniquet to diagnose the presence of compromised venous outflow.81–84 A normal IPG test was helpful to rule out the presence of the highest-risk DVT condition, a large proximal DVT.81
Plethysmographic techniques do not provide anatomically specific information; they only suggest the diagnosis of DVT. Delayed venous outflow after cuff release indicates a more central obstruction, but the finding is not specific for the location or cause of the outflow obstruction. Plethysmography cannot reliably detect distal (calf vein) or nonocclusive thrombi. Other physiologic or physical abnormalities can produce false-positive tests—including obesity, pregnancy, congestive heart failure, external venous compression, and chronic DVT—but these conditions may also be risk factors for acute DVT. Lack of patient cooperation, arterial occlusive disease, and low-ambient temperatures may also contribute to inadequate venous plethysmography results. Based on these limitations, venous plethysmography is no longer appropriate for the evaluation of suspected acute DVT, although it may have an ancillary role for physiologic evaluations in the vascular laboratory.
Doppler Flow Detection
The use of continuous-wave or pulsed Doppler ultrasound for flow detection was another early technique to assess for venous thrombosis.85–92 The absence of Doppler-detectable flow in an insonated segment was used as a criterion for the diagnosis of DVT, but the early Doppler ultrasound systems lacked the imaging capability needed to allow the technologist to be certain about which vein was being evaluated, and they did not provide information about anatomic abnormalities.
The early techniques of Doppler flow detection were insensitive to calf vein thrombi and to limited or nonocclusive thrombus. Performance and interpretation of these examinations required highly experienced personnel, as Doppler findings alone were subjective. False-positive examinations resulted from chronic disease interpreted as acute thrombosis or from the interpretation of low-amplitude signals as indicative of thrombosis, though this might have been a nonpathologic finding in calf veins, in obese patients, or with excessive Doppler probe pressure impairing venous flow.93,94 A negative Doppler flow study in a patient with low suspicion for acute DVT was considered sufficient to rule out the diagnosis, but venography was needed when the noninvasive study was equivocal or when the clinical circumstances strongly suggested the diagnosis.95–97
Ultrasound Imaging Techniques
First described by Talbot in 1982,4 B-mode ultrasound imaging for diagnosis of lower extremity venous thrombosis has proved to be practical and widely adopted. DVT is diagnosed by direct visualization of thrombus in the veins. Unlike normal veins that easily collapse with extrinsic compression, thrombosed segments appear dilated and do not collapse with the application of manual (transducer) pressure over the imaged segment.
Early validation studies comparing venous ultrasonography to the reference standard of contrast venography identified shortcomings in the use of B-mode imaging as a stand-alone modality, as imaging of deeper structures was limited and some normal venous segments might not collapse with moderate probe pressure (e.g., femoral vein at the level of the adductor hiatus). Venous compressibility can also be limited by obesity, edema, or tenderness. It was soon recognized that duplex scanning, the combination of B-mode imaging and Doppler-derived information about the presence and nature of venous flow in the interrogated segments, improved the accuracy of the examination.98
Duplex scanning was rapidly adopted for diagnosis of DVT due to its clear advantages over the more cumbersome indirect plethysmographic methods. A review by White et al99 found the sensitivity of duplex ultrasound for detecting proximal limb thrombi in four well-designed studies (1980–1988) was 92% to 95%, with reported specificity of 97% to 100%. Similar findings were noted in nine other studies that had minor methodologic flaws. It was also recognized that ultrasound imaging could identify a nonthrombotic cause of leg symptoms in 5% to 15% of cases.
Advances in imaging technologies have further improved the accuracy and ease of venous studies for DVT. The addition of color flow Doppler imaging facilitates vessel identification in large limbs and helps with calf vein evaluation. All examination protocols, however, utilize compression as a basic element of the study.
In a clinical practice guideline from the American Thoracic Society, the sensitivity and specificity of various ultrasound techniques for DVT diagnosis were reviewed.100 In those papers reporting results from appropriately designed prospective studies with venographic correlation, the sensitivity of real-time B-mode (compression) ultrasound for suspected proximal DVT in symptomatic patients was 89% to 100%, with reported specificity ranging from 86% to 100%. Studies of venous duplex scanning reported sensitivities of 88% to 97% and specificities of 80% to 100%. Reports of the accuracy of color flow Doppler ultrasound indicated a sensitivity of 95% to 96% and specificity of 99% to 100%.
Because of its accuracy, low cost, and noninvasive nature, venous duplex scanning is now the diagnostic method of choice for DVT.101 However, a potentially unwanted effect of the test being so safe, inexpensive, and widely available is its overuse. Providers may tend to request the examination unnecessarily for irrelevant clinical signs and in the absence of any evident DVT risk factors.102,103
Point of Care Ultrasound Imaging for DVT
The availability of point of care ultrasound (POCUS) systems in emergency rooms, intensive care units, and other clinical settings can facilitate testing for DVT, but with potential for diagnostic errors.104 With appropriate training, however, it appears that nonvascular specialist physicians can diagnose femoropopliteal DVT with accuracies approaching those of vascular laboratory professionals.105 The two-point compression ultrasound examination of the common femoral and popliteal veins as a screening test for acute proximal lower extremity DVT is discussed in Chapter 27. It is anticipated that venous POCUS applications will become increasingly important in clinical practice.
ADVANCED IMAGING MODALITIES
Although venous duplex ultrasonography has become the standard for detection of acute DVT, adjuvant modalities have roles in selected cases.106
Magnetic Resonance Imaging
Magnetic resonance venography (MRV) can be used for evaluating the pelvic veins.107–111 Thrombus is evident either as a venous segment without flow or as a discrete filling defect in the vein lumen with a flow-based approach such as time-of-flight or gradient-focused rapid imaging. MRV is most likely to be useful when venous thrombi are located above the inguinal ligament or when extrinsic compression of the iliac veins mimics the signs and symptoms of DVT, but MRV has also been found useful for femoropopliteal and calf DVT evaluations, with accuracy reported to be greater than 90% in the few series studying this application.112,113 MRV is contraindicated for patients with pacemakers, and those with claustrophobia do not tolerate the procedure well.
Computed Tomographic Venography
Computed tomography (CT) can also be used to diagnose lower extremity DVT. Similar to MRV, computed tomographic venography (CTV) was originally used for evaluation of the iliac veins.114,115 There are potential advantages of CTV over iliac vein venography, as CT scanning can identify sources of extrinsic venous compression, as well as diagnose thrombosis. CTV does not, however, yield high-resolution anatomic details of the vein lumen, and CTV may not detect subtle changes from prior episodes of DVT.
CTV obtained in conjunction with CT pulmonary angiography has been evaluated for the diagnosis of DVT in thigh and calf veins.116–118 Delayed (3-minute) imaging shows good diagnostic performance when compared to contrast venography and ultrasound imaging. This approach offers the potential advantage of screening for both DVT and PE with a single examination, but the accuracy and cost-effectiveness of this approach are not well-established.
DUPLEX SCANNING FOR DVT: ISSUES FOR THE VASCULAR LABORATORY
Bilateral Versus Unilateral Examinations
The noninvasive nature of duplex scanning makes it a suitable test to use for screening or evaluation of asymptomatic limbs. Routinely scanning both extremities, however, appears to be unnecessary in all cases,119 and unilateral studies are commonly done in many vascular laboratories.120
A unilateral approach is justified when a prothrombotic state is not suspected, when there are no systemic symptoms or indications of PE, and when the study is done as a follow-up to assess a limb with known DVT (Fig. 19.1). When outpatients are referred for evaluation of unilateral lower extremity symptoms, it is rare to find unsuspected thrombus in the asymptomatic contralateral limb, unless the patient has risk factors for thrombosis. The incidence of unilateral thrombus in an asymptomatic extremity in an otherwise healthy individual approximates 1%.121 Outpatients are at risk for clinically silent thrombus in an asymptomatic extremity if they have coagulopathic risk factors such as active malignancy, recent operation or injury, prolonged bed rest, or pregnancy.122 For these patients, a bilateral study is recommended. Inpatients and patients with bilateral symptoms warrant a bilateral evaluation.123,124
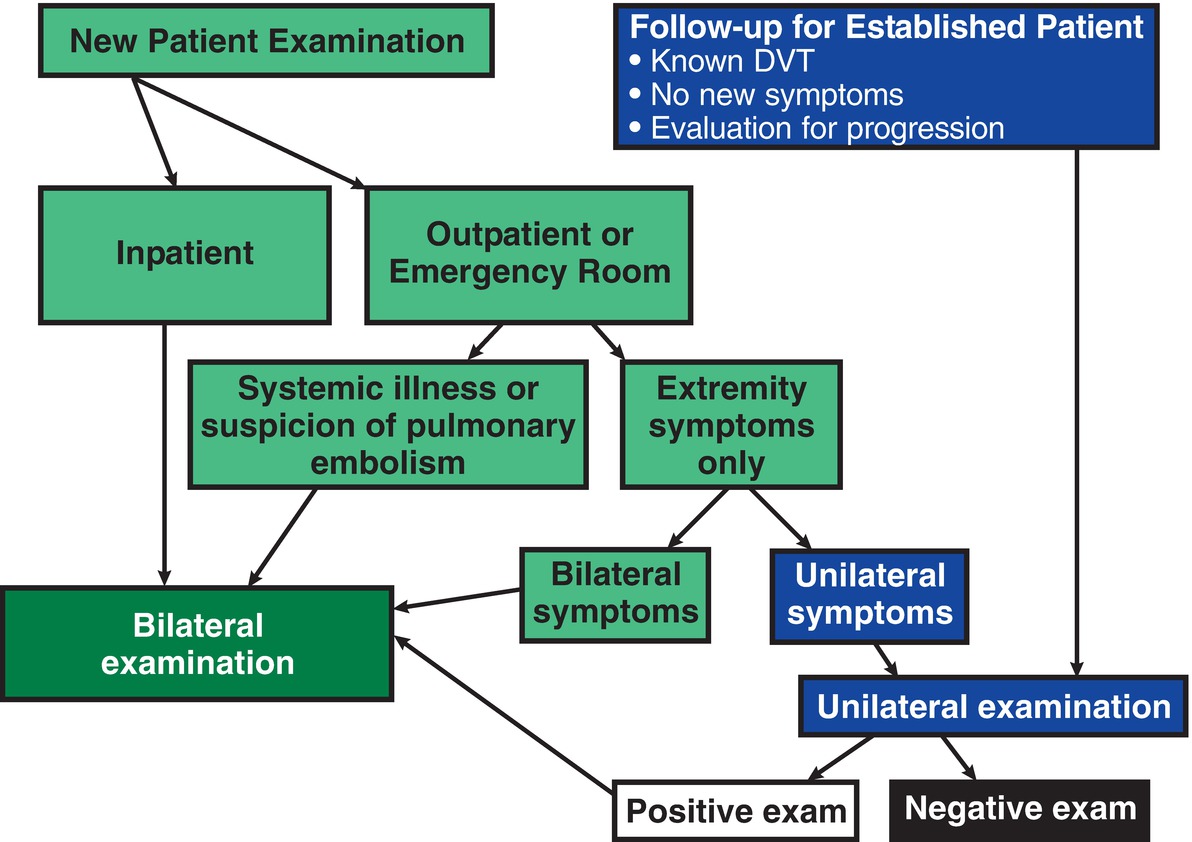
FIGURE 19.1 Algorithm for selecting a unilateral versus bilateral venous duplex examination.
Calf Vein Thrombosis
The importance of calf vein thrombosis has been a subject of some controversy. Though isolated calf vein thrombi do not cause clinically relevant PE, these patients are at risk for thrombus progression to involve the femoropopliteal veins. Symptomatic calf vein thrombi are associated with progression or recurrent thrombosis in 15% to 20% percent of patients within 3 months.125,126 Calf vein thrombosis can be a predictor of thromboembolic risk for which anticoagulation may be indicated.61 However, routine treatment of all patients with calf DVT does risk overuse of anticoagulation.127 Factors that appear important in stratifying risk include whether thrombus is confined to the distal limb veins (isolated calf DVT) or also involves the veins of the proximal limb128–130 and whether the DVT was a first episode of VTE or a second or subsequent episode.131,132
In a meta-analysis that included two randomized controlled trials (RCTs) and six cohort studies, PE was significantly less frequent in patients with calf DVT who received anticoagulation compared to those who were not treated, with a trend toward fewer deaths with anticoagulation. When the analysis was limited to RCTs, the benefit of anticoagulation for PE was no longer statistically significant, but the benefit for preventing thrombus progression persisted.133 Thus, treatment options for patients with calf DVT include either routine anticoagulation or selective anticoagulation if a follow-up duplex scan demonstrates thrombus progression. The ninth edition of the ACCP guidelines recommends serial imaging for 2 weeks, rather than initial anticoagulation, for isolated acute calf vein thrombosis without severe symptoms and when the patient does not have risk factors for thrombus propagation.134 Patients at increased risk include those who have recently had orthopedic procedures, patients with malignancy, and those who are immobile.135
Color flow duplex scanning has been found to be reliable as a method for evaluating calf veins for DVT, but calf veins may be poorly imaged in obese patients or those with chronic edema. Calf vein thrombosis is common in patients who have acute DVT and often occurs as an isolated finding.136 The peroneal and posterior tibial veins are involved in the majority of cases; thrombi occur much less frequently in the anterior tibial veins.
Calf Muscle Venous Thrombosis
Thrombosis of veins within the gastrocnemius or soleus muscles of the calf either can be an isolated finding or can be found in combination with proximal DVT. Calf muscle venous thrombosis (CMVT) can be associated with localized calf tenderness. In rare cases, there can be propagation and subsequent PE, but isolated CMVT, compared to thrombosis of multiple calf veins, appears to have a lower risk of extension, and if extension is not observed within 2 weeks, it is unlikely to occur.134,137–139
Treatment recommendations for isolated CMVT vary. Some studies have shown reduced risk of thrombus progression or PE with anticoagulation therapy, but others showed no benefit.139 For low-risk patients without active cancer (or other prothrombotic conditions), anticoagulation is not necessary. Patients with transient risk factors, however, should be re-evaluated with duplex scanning 1 week after diagnosis to confirm thrombus resolution or absence of progression.140
Diagnosis of Recurrent DVT
Recurrent thromboembolic events after an initial episode of DVT are relatively frequent.141 Patients with a treated DVT may be referred for repeat studies if they have new symptoms of pain and swelling. Differentiating postthrombotic symptoms from recurrent acute DVT is facilitated if a new baseline is established after a course of anticoagulation has been completed.
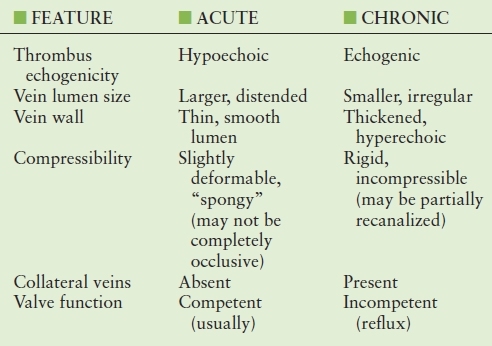
The predictive value of sonographic features for establishing the age of a deep vein thrombus is not well-established. The sonographic features that distinguish acute from chronic thrombus need to be considered in the context of the patient’s clinical presentation, and it should be emphasized that no imaging feature can be considered reliable in all circumstances. The features of chronic DVT can be confused with acute thrombosis (or “acute-on-chronic DVT”). Understanding the ambiguity of the ultrasound diagnosis can be important, as it is not uncommon for a patient with PTS (including pain and swelling) to be referred to the vascular laboratory to “rule out DVT.”
The late appearance of a venous segment can vary significantly after acute DVT. Some segments may remain chronically occluded, or there may be complete or partial recanalization. Thrombus echogenicity can vary, and the appearance of thrombus can be altered by changes in the image-processing settings, and it can be affected by image depth and transducer selection. Incomplete compressibility, a feature of acute nonocclusive DVT, can also be observed with chronic DVT when there is partial recanalization of a previously occluded segment.142 Veins with acute thrombus typically appear larger than normal veins, and veins with chronic DVT are generally smaller than normal veins, but the ranges of normal and abnormal vein diameters overlap, so size alone cannot be used for the diagnosis of acute versus chronic DVT, except perhaps at “extreme” diameters.143
Only when the extent of residual thrombus has been established can the diagnosis of acute-on-chronic thrombosis be made with ultrasound imaging. When a subsequent examination shows thrombosis of previously clear segments, the diagnosis can be made with confidence. On the other hand, visualization of chronic thrombus with no change from prior examinations suggests venous insufficiency from PTS as the cause for recurrent or persistent symptoms. Other features of PTS may include venous reflux (see Chapter 21).
A negative d-dimer test can be used to confirm the absence of new acute thrombosis.144 For the evaluation of patients with suspected recurrent DVT when a prior ultrasound is not available for comparison, d-dimer testing with a high-sensitivity assay is the recommended first step.145 As with the initial diagnosis of acute DVT, the finding of an elevated d-dimer is nonspecific, and the abnormal result cannot be taken as an indication that there is recurrent acute thrombosis.
Guiding the Duration of Anticoagulation for DVT
While 3 to 6 months of therapy is a common clinical recommendation for acute DVT, the optimal duration of anticoagulation may differ for individual patients. Anticoagulation continues to reduce the risk of recurrent VTE for as long as it is used, although the absolute risk of recurrent VTE declines over time.146 While the risk of recurrent VTE is reduced with long-term antithrombotic therapy, this benefit must be weighed against the risk of bleeding, costs, and patient preferences. Patients with an increased risk for bleeding on long-term anticoagulation therapy are those with advanced age, previous bleeding episodes, renal or hepatic impairment, treatment with high-intensity anticoagulation, and concomitant therapy with antiplatelet agents. Because anticoagulation risks persist as treatment benefit declines, the overall efficacy of continued therapy decreases over time.
Clinical prediction rules have been developed to estimate risks of VTE recurrence after a course of anticoagulation for acute DVT, as well as to guide the duration and intensity of continued therapy. The presence of residual thrombus after the completion of a course of anticoagulant therapy was identified as a potential risk factor for recurrent DVT.22,141,147–149 It has also been identified as a risk factor for other adverse outcomes, including death, suggesting that failure of thrombus resolution may be a marker of vascular dysfunction.22 In some studies, absence of residual thrombus after treatment was associated with a lower risk for DVT recurrence after discontinuation of anticoagulation.141,147
The AESOPUS trial evaluated treatment of DVT, with anticoagulation duration based on the absence or persistence of residual thrombi on ultrasonography.23 After a 3-month period of anticoagulation, this trial randomized DVT patients to fixed-duration anticoagulation (no further anticoagulation for secondary thrombosis and an extra 3 months for unprovoked thrombosis) or flexible-duration, ultrasound-guided anticoagulation. In the ultrasound-guided treatment group, no further treatment was given if the veins had recanalized, but other patients continued anticoagulation for up to 9 months for secondary DVT and up to 21 months for unprovoked thrombosis. The rate of confirmed recurrent VTE during 33 months of follow-up was 17% in the patients allocated to fixed-duration anticoagulation and 12% for patients allocated to flexible-duration, suggesting that tailoring the duration of anticoagulation on the basis of ultrasound findings might reduce the rate of recurrent VTE in adults with proximal DVT. However, an important limitation of this study was the lack of a comparison group without residual thrombus that was treated with continued anticoagulation.
The PROLONG study evaluated patients with d-dimer testing 1 month after the discontinuation of anticoagulation for an episode of unprovoked proximal DVT. Those with a normal d-dimer level did not resume anticoagulation, while those with an abnormal d-dimer were randomized to either resume anticoagulation or continue without therapy. Patient follow-up averaged 1.4 years. For those with an elevated d-dimer, the adjusted hazard ratio for recurrent VTE was 4.3 for patients who were not anticoagulated compared to those who were.24 Further analysis of data from this trial suggested that the effect of a persistently elevated d-dimer on recurrent VTE risk was more significant than the persistence of sonographically identified residual thrombosis.25
It appears that any component of risk associated with residual venous obstruction (thrombus) as a predictor for recurrent VTE declines over time. In a meta-analysis of ten prospective studies including 2527 patients with a first unprovoked DVT, residual venous obstruction was found in 55% after a median of 6 months. Recurrent VTE occurred in 16% during a median follow-up of 23.3 months. When found 3 months after the acute DVT, residual venous obstruction was associated with increased recurrent VTE risk (hazard ratio 2.2), but there was no significant increase in recurrent VTE risk associated with the finding of residual thrombus after 6 months. This suggests that the presence of persistent chronic thrombus is not an indicator of an ongoing prothrombotic condition.26 In general, ultrasound findings of residual venous thrombus should not be used as a primary or stand-alone basis for decisions about the duration of anticoagulation for DVT, but they should be considered in the clinical context.
DUPLEX EVALUATION
Instrumentation
Lower extremity deep veins are well-visualized by ultrasound in most patients and generally easy to evaluate for thrombosis. A full-featured, high-resolution duplex scanner enables the technologist to perform a quality examination and to identify subtle segments of abnormalities or thrombosis, even in large patients. Transducer selection depends on the depth of the veins scanned and the approach used. A 5- to 10-MHz linear array transducer is often sufficient for a complete lower extremity venous examination. However, a 3- to 5-MHz curved linear transducer may be necessary when evaluating deeply located vessels such as the inferior vena cava (IVC) and iliac veins or to evaluate obese patients or edematous extremities.
Patient Preparation and Positioning
No special patient preparation is needed before a lower extremity venous duplex examination from the level of the groin to the distal leg, but it is helpful to have the patient fast for 4 to 6 hours if the IVC or iliac veins are to be evaluated to reduce overlying bowel gas that may interfere with imaging. The standard examination is performed with the patient supine and the bed or gurney adjusted to provide moderate reverse Trendelenburg (head up, feet down) positioning. This promotes venous filling in the lower extremities and facilitates ultrasound visualization.
To facilitate a thorough examination of all relevant lower extremity veins (Table 19.5), the extremity being evaluated should be completely exposed to the ankle, and the limb should be externally rotated at the hip and slightly bent at the knee. Positioning the patient as close to the technologist as possible helps the technologist use ergonomically correct body mechanics. However, some patient factors can make patient positioning and scanning difficult, including paraplegia, recent extremity surgery, trauma, or the need to perform the examination in the intensive care unit. Assistance with optimal patient positioning in such cases may facilitate use of scanning positions that reduce stresses on the back, shoulder, and wrist of the technologist.
TABLE 19.5 Nomenclature of the Deep Veins of the Lower Extremity

Adapted from Caggiati A, Bergan JJ. Gloviczki P, et al. Nomenclature of the veins of the lower limbs: an international interdisciplinary consensus statement. J Vasc Surg 2002;36(2):416–422; Caggiati A, Bergan JJ, Gloviczki P, et al. Nomenclature of the veins of the lower limb: extensions, refinements, and clinical application. J Vasc Surg 2005;41(4):719–724. ICAVL Standards for Accreditation in Noninvasive Vascular Testing. Part II. Vascular Laboratory Operations: Peripheral Venous Testing. 2007; http://www.icavl.org/icavl/pdfs/venous2007.pdf. Accessed June 30, 2009.
IAC, Intersocietal Accreditation Commission
B-Mode Imaging
Normal Findings
B-mode imaging is used to perform venous compression maneuvers and for direct visualization of intraluminal abnormalities. Vein compression is performed by manual application of enough downward probe pressure to coapt the vein walls without compressing the adjacent artery (Fig. 19.2). A patent, thrombus-free vein will demonstrate complete vein wall coaptation with probe compression along its entire course, with no intraluminal echoes identified (Fig. 19.3). Compressions should be performed in a transverse (cross-sectional) view to make sure that the entire vessel is visualized during the maneuver. If a longitudinal view is used, the probe can slip off the vessel, giving a false impression of complete vein wall compression. Veins should be evaluated for compressibility in segments approximately one probe width apart.
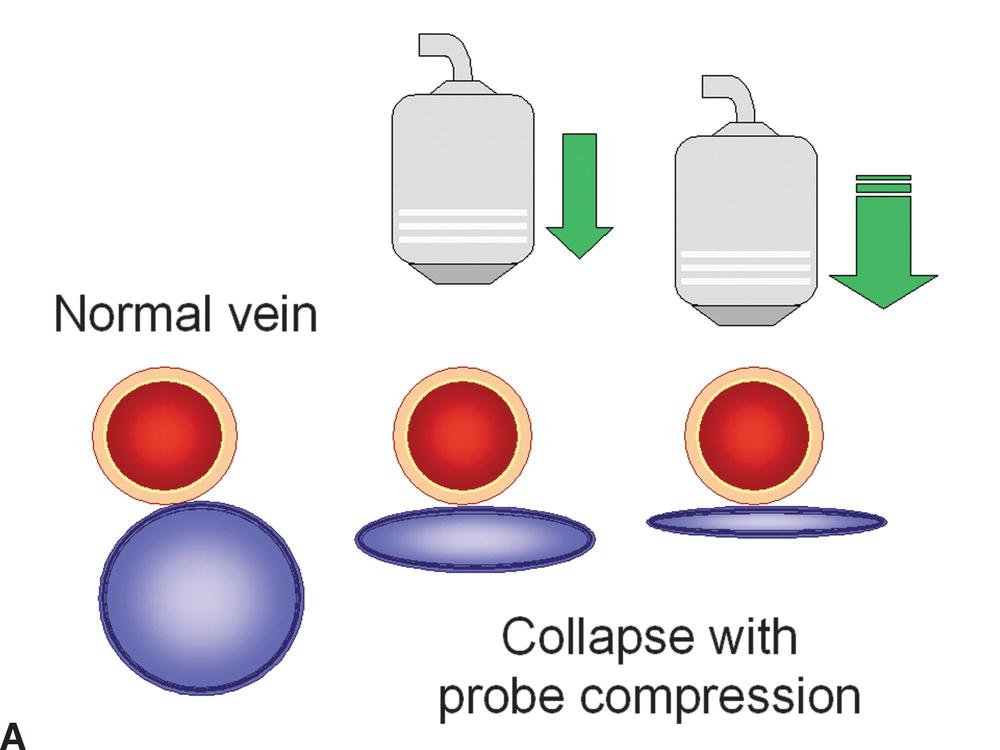
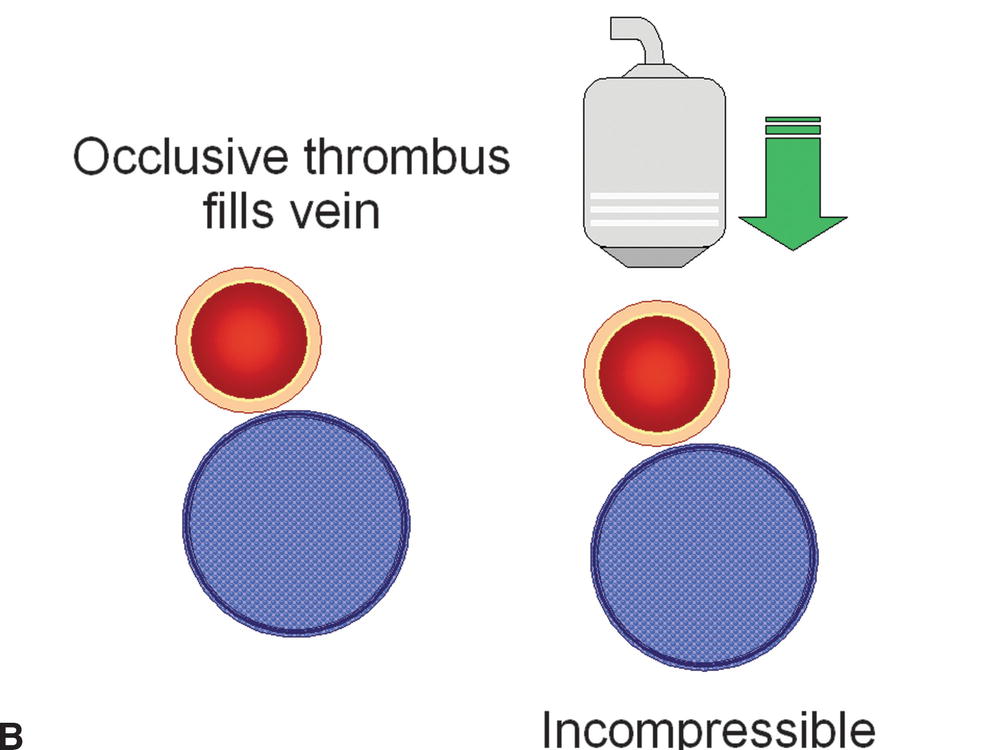
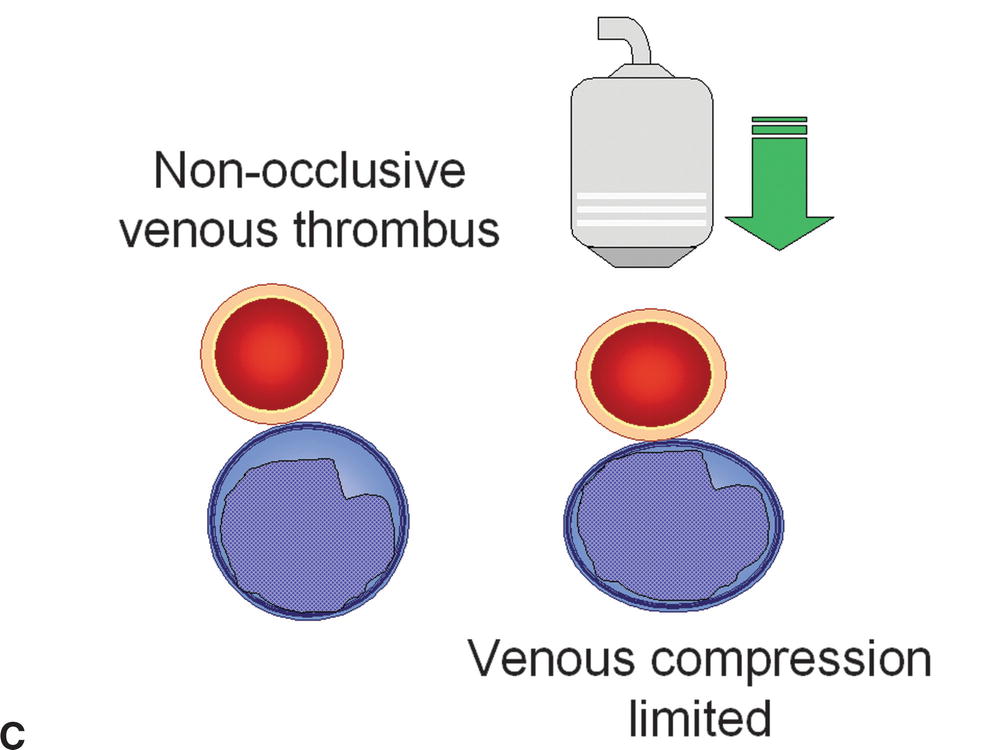
FIGURE 19.2 The presence or absence of thrombus within an imaged vein can be confirmed with probe compression in a transverse view. A,Increasing the pressure of the transducer on the examined vein will result in collapse of the normal thin-walled and low-pressure vein. B,If the vein is filled with thrombus, it will be incompressible. C,Nonocclusive thrombus may allow some but limited compression of the vein.
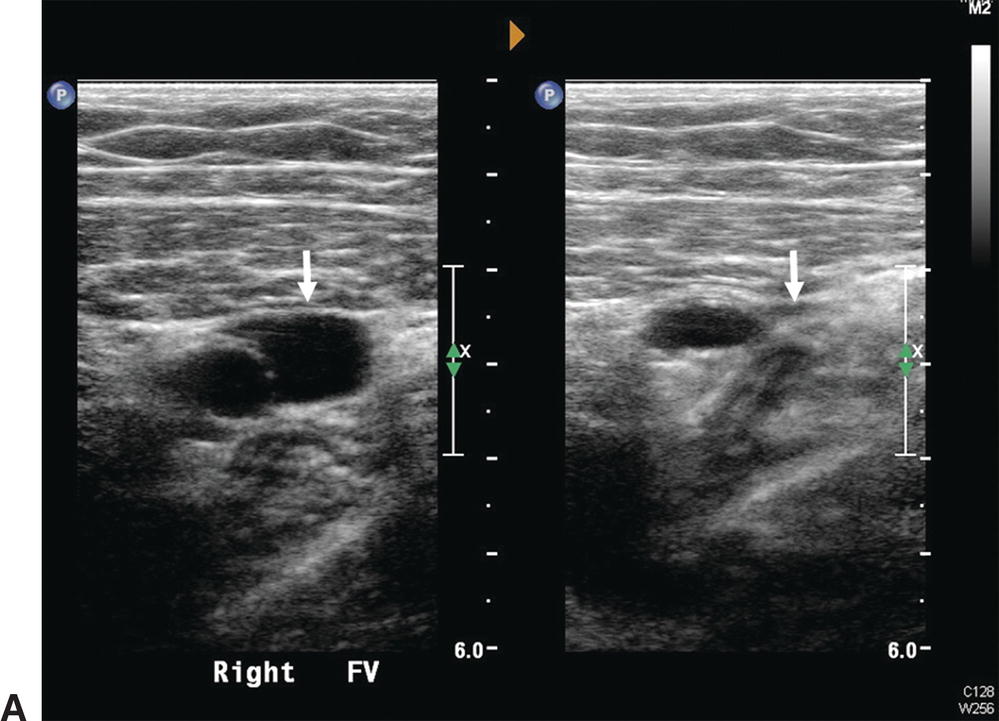
A,Normal femoral vein in transverse view (arrows): Left, without probe compression; Right, with compression and vein walls completely coapted. The adjacent superficial femoral artery is partially compressed. B,Normal femoral vein in longitudinal view is seen to have a smooth surface, thin wall, and hypoechoic lumen. A normal, spontaneous, phasic flow pattern is demonstrated.
Abnormal Findings
Vein incompressibility reliably indicates the presence of intraluminal thrombus. In the presence of an acute thrombus, the vein will be incompressible and dilated; the vein diameter is usually larger than that of the adjacent artery, and intraluminal echoes are typically anechoic or hypoechoic (Fig. 19.4). Acute thrombus may be visualized adherent to the vein wall, or it may demonstrate mobility such as a “free-floating thrombus” or “thrombus tail” (Fig. 19.5). Although the risk of causing thromboembolism is probably small,27 caution is advised in performing compression maneuvers over a nonoccluding mobile thrombus, as there have been reports of acute embolization of loosely attached thrombi during examinations.28–30 As previously discussed, it is not possible to reliably determine the age of a venous thrombus by ultrasound; however, some general features that help to distinguish between acute and chronic thrombus are listed in Table 19.6.
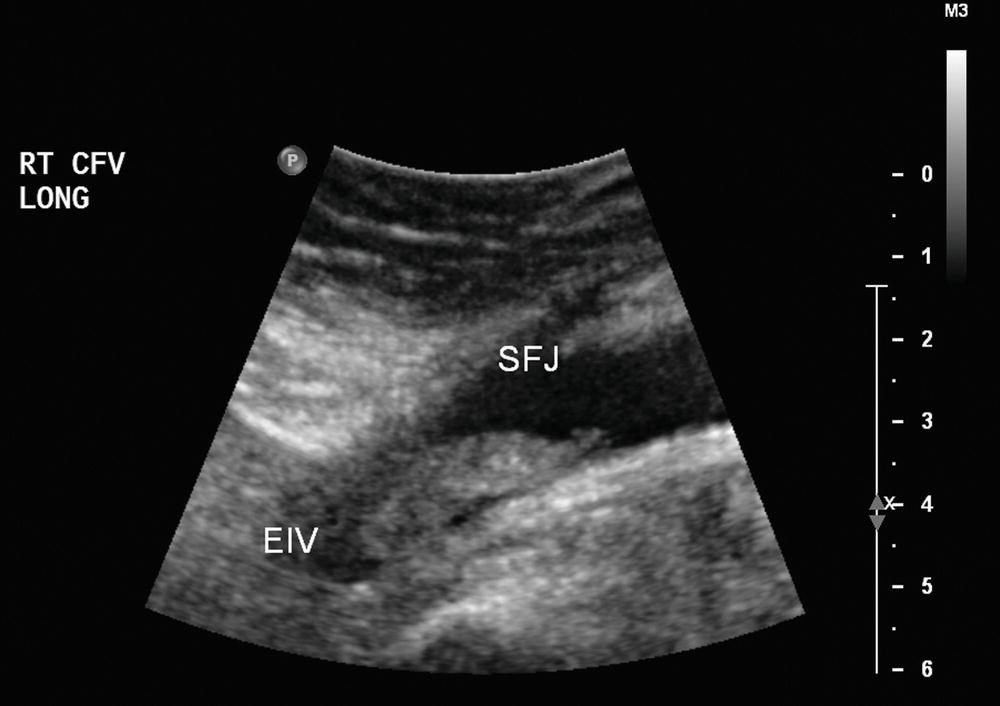
FIGURE 19.4 Thrombus in the common femoral vein (CFV). In some cases, it may be difficult to determine the cephalad extent of iliofemoral thrombus, as iliac veins are deep in the pelvis and may be obscured by bowel gas. (EIV, external iliac vein; SFJ, saphenofemoral junction.)
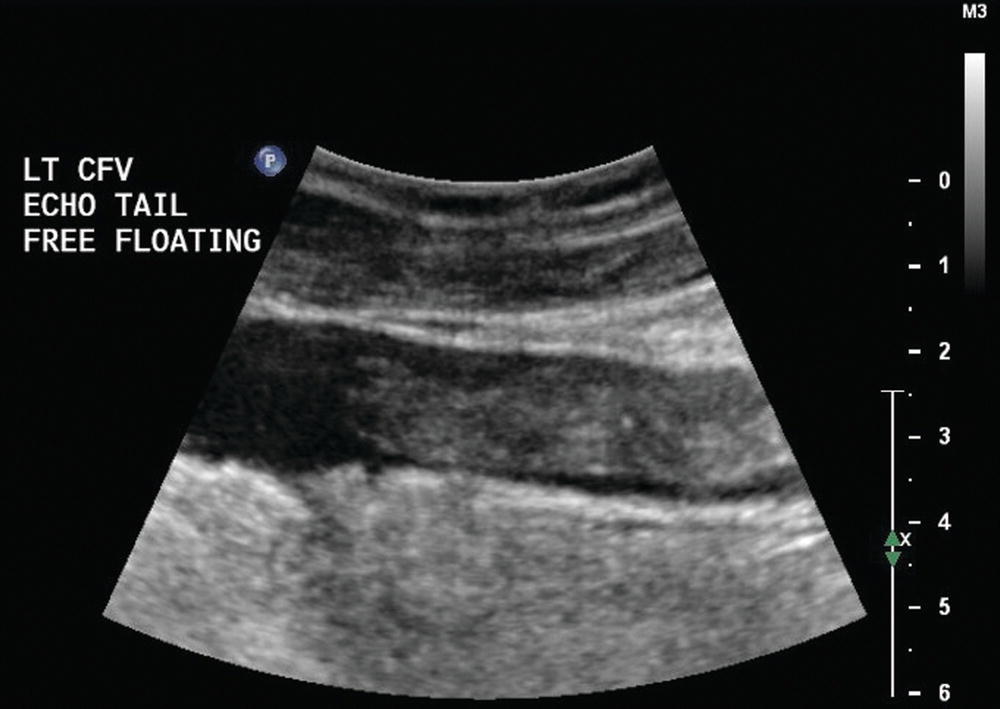
FIGURE 19.5 Homogeneous moderately echoic material filling the lumen of this common femoral vein (CFV) is typical for the B-mode ultrasound appearance of acute deep vein thrombosis. In some cases of thrombosis in large veins, the cephalad end of the thrombus may not be attached to the vein wall (“free floating”) and it may be seen to move.
TABLE 19.6 Ultrasound Features that may help to Distinguish between Acute and Chronic Venous Thrombus
Veins may be difficult to completely collapse with probe compression when they are deeply situated or located within relatively incompressible tissues, such as in the pelvis or adductor canal area above the knee. Evaluation with pulsed Doppler or color flow Doppler imaging can confirm the patency of venous segments that do not easily collapse with compression. Outflow obstruction with resulting venous hypertension can make a vein appear dilated, incompressible, and without spontaneous flow. In this unusual circumstance, the segment may be incorrectly reported as occluded.
Color Flow and Pulsed Doppler
Normal Findings
Color flow Doppler and pulsed Doppler spectral waveform analysis are used to evaluate venous flow characteristics. Color flow Doppler is helpful in confirming patency of venous segments that are incompletely evaluated with B-mode imaging (Fig. 19.6). This helps to differentiate between occluding and nonoccluding thrombus and identify anechoic thrombus by visualization of flow around it (Fig. 19.7). The color velocity scale (pulse repetition frequency or PRF) should be at a low setting to detect low-velocity venous flow.
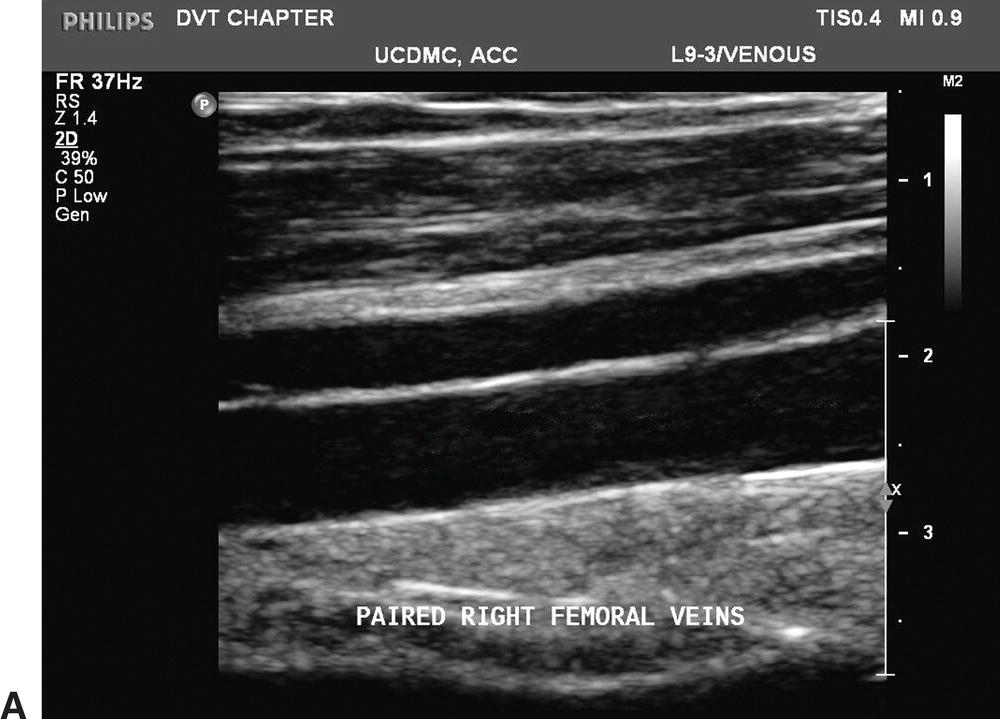
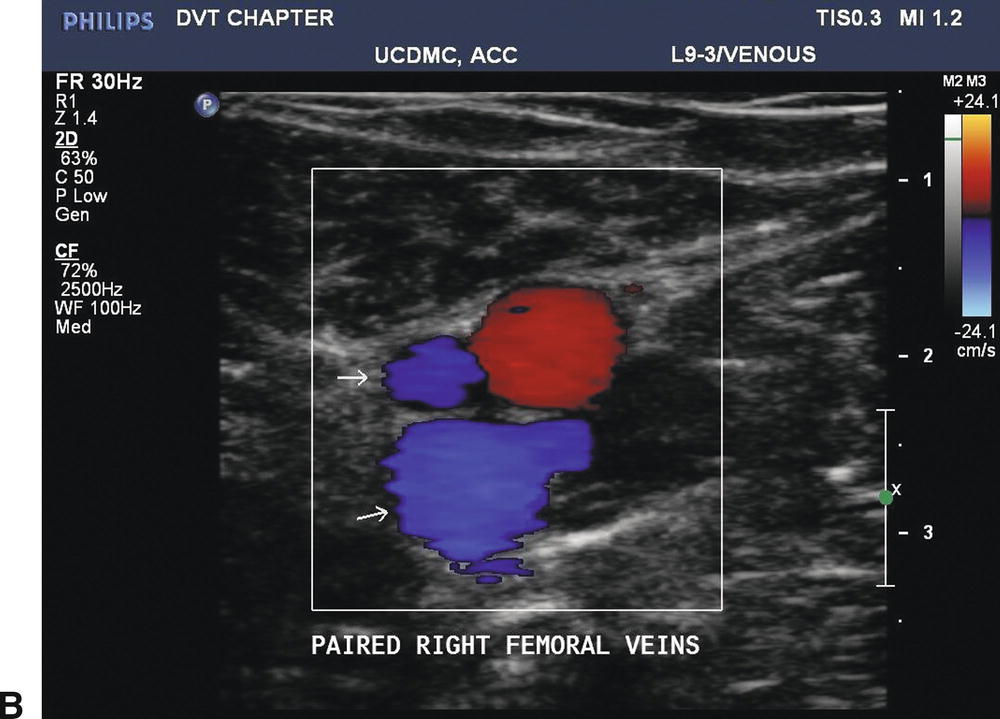
FIGURE 19.6 The deep veins below the knee are paired, but paired veins in the proximal lower limb are a common variant. A,Longitudinal B-mode image of paired femoral veins. B,Color Doppler imaging in the transverse plane shows the paired femoral veins to be unequal in size, a typical finding when two femoral veins are present.
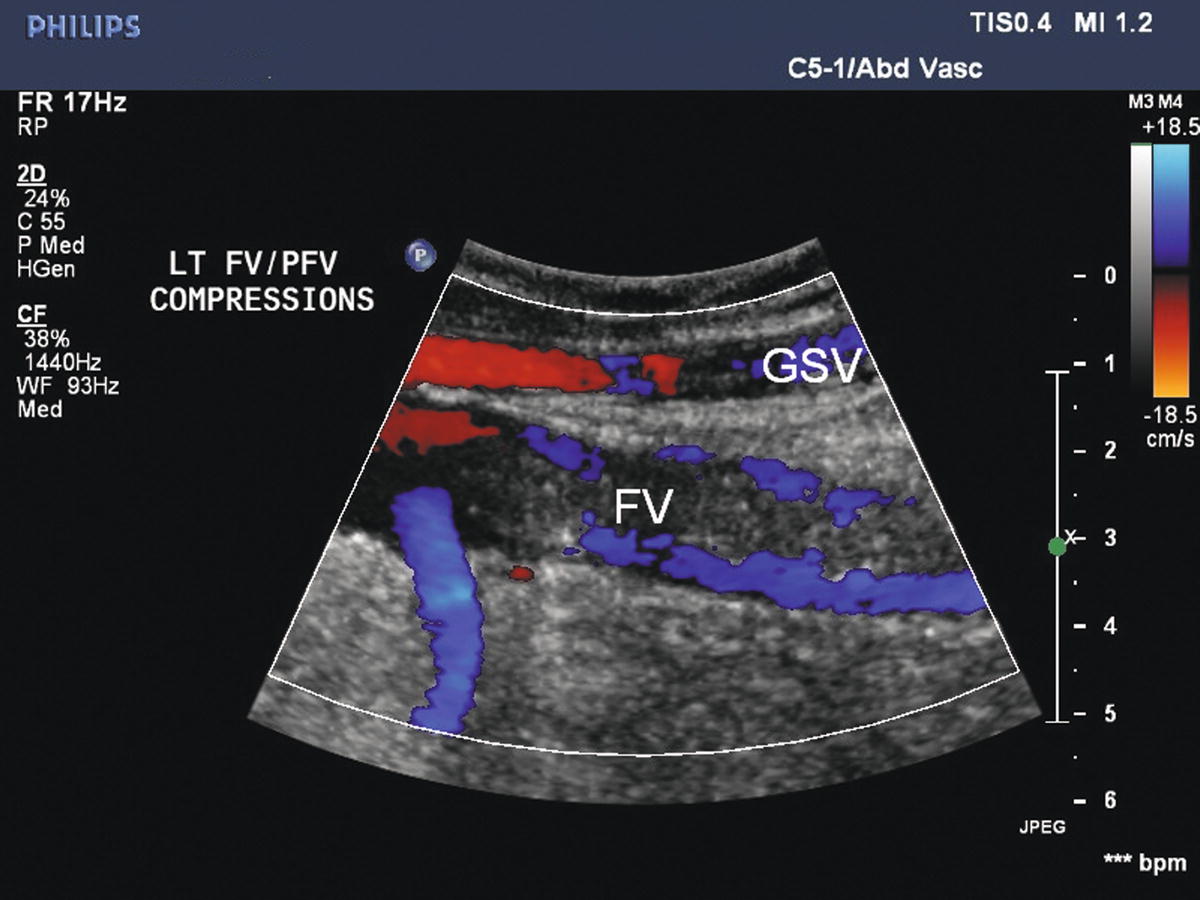
FIGURE 19.7 Acute thrombus may not be tightly attached to the vein wall. Color Doppler demonstrates flow around acute intraluminal thrombus in the femoral vein (FV) with distal thigh compression (augmentation). The great saphenous vein (GSV) is patent.
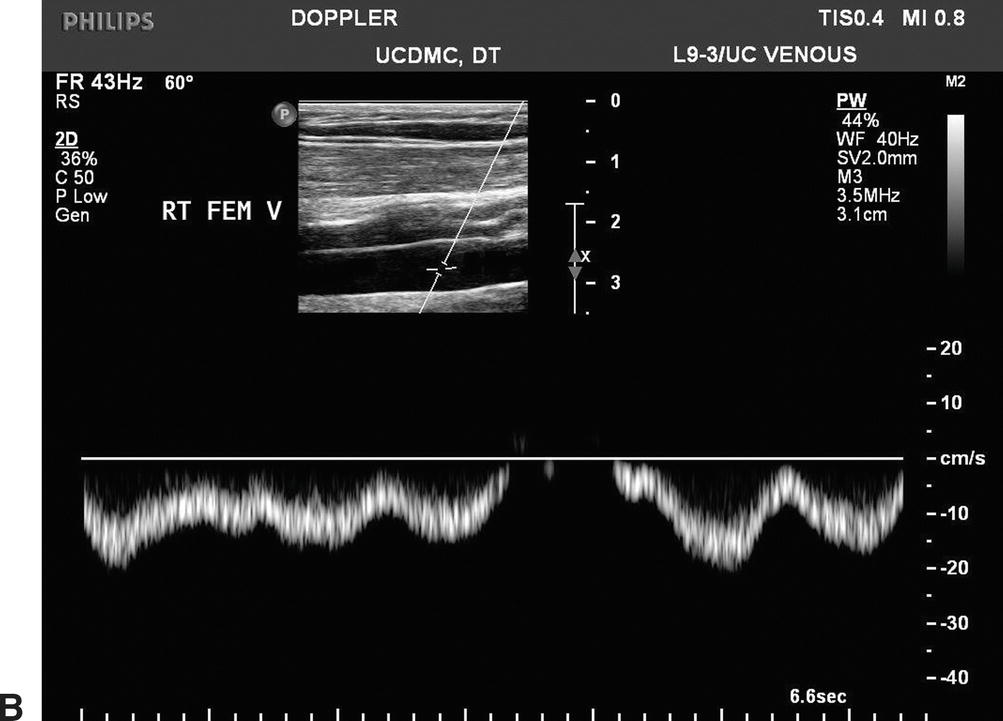
Doppler spectral waveforms should indicate a flow pattern that is spontaneous (present without augmentation) and phasic (varying with the respiratory cycle), as shown in Figure 19.8. By convention, flow waveforms are displayed with normal antegrade venous flow (flow toward the heart) below the baseline. Respiratory phasicity represents the normal variations in lower extremity venous flow that occur in response to changes in abdominal pressure as the diaphragm moves up and down. Venous flow patterns become less phasic and spontaneous with distance from the heart, so these features may not be prominent in the tibial veins. Comparing venous flow waveforms from the left and right sides is helpful in distinguishing normal variation from pathologic findings. Asymmetric flow patterns suggest a unilateral abnormality.
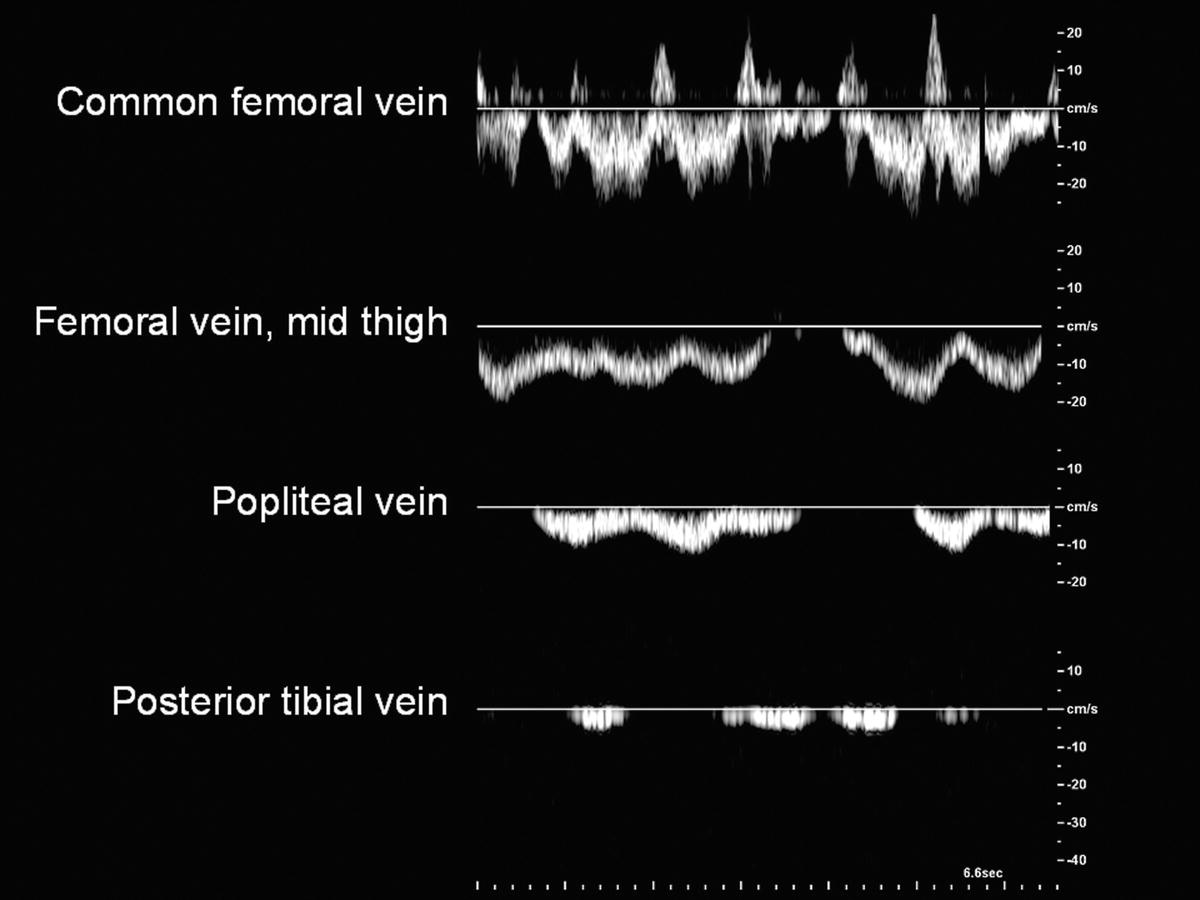
FIGURE 19.8 Normal flow in the lower extremity veins is spontaneous (occurring without calf pump function or augmentation maneuvers) and phasic with respiration. Observed flow patterns are different in various locations, with less spontaneous flow in the small veins of the distal limb.
Distal augmentation maneuvers are performed with manual calf compressions while interrogating the common femoral, femoral, and popliteal vein segments. Foot compressions can be used to evaluate the tibial and peroneal veins. A sharp “spike” of antegrade venous flow in response to distal limb compression suggests patency between the level of compression and the site of Doppler interrogation (Fig. 19.9). Blunted or absent augmentation of flow with distal limb compression suggests obstruction below the level being interrogated. Distal augmentation maneuvers are not recommended when an acute deep vein thrombus has already been identified.
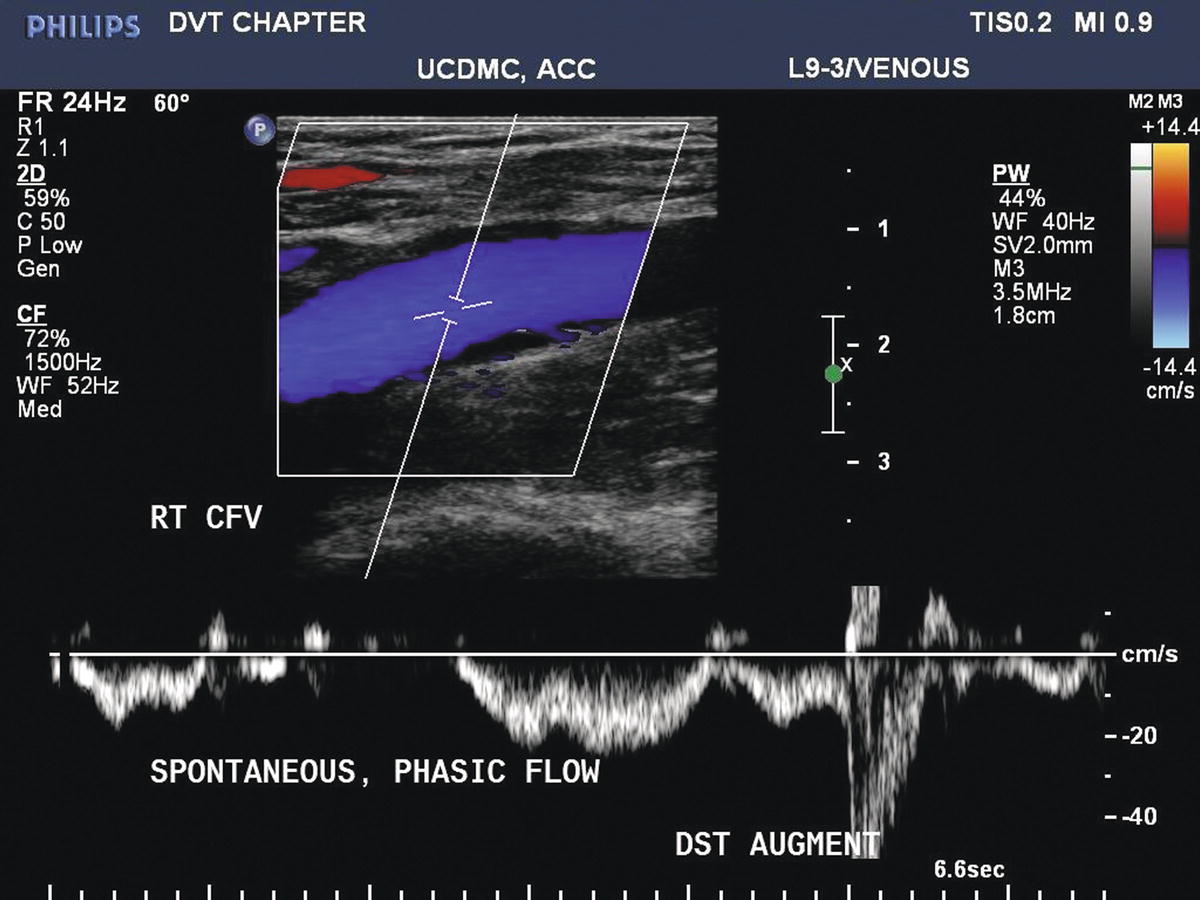
FIGURE 19.9 Color Doppler image of a normal common femoral vein (CFV). Flow is phasic with respiration. Transient reversal of flow is normally observed in a supine subject, as retrograde flow velocities with respiration may be insufficient for vein valve closure. Augmentation of flow (a brief increase in antegrade flow velocity) can be demonstrated with manual compression of the limb (DST AUGMENT).
The Valsalva maneuver or proximal compression maneuvers may be used to test valvular competency (Fig. 19.10); however, a complete examination for reflux is not required as part of a duplex examination for acute DVT. The evaluation of chronic venous insufficiency is discussed in Chapter 21. Valvular incompetence may be associated with chronic DVT, and it is a feature of the PTS (Figs. 19.11, 19.12, and 19.13).
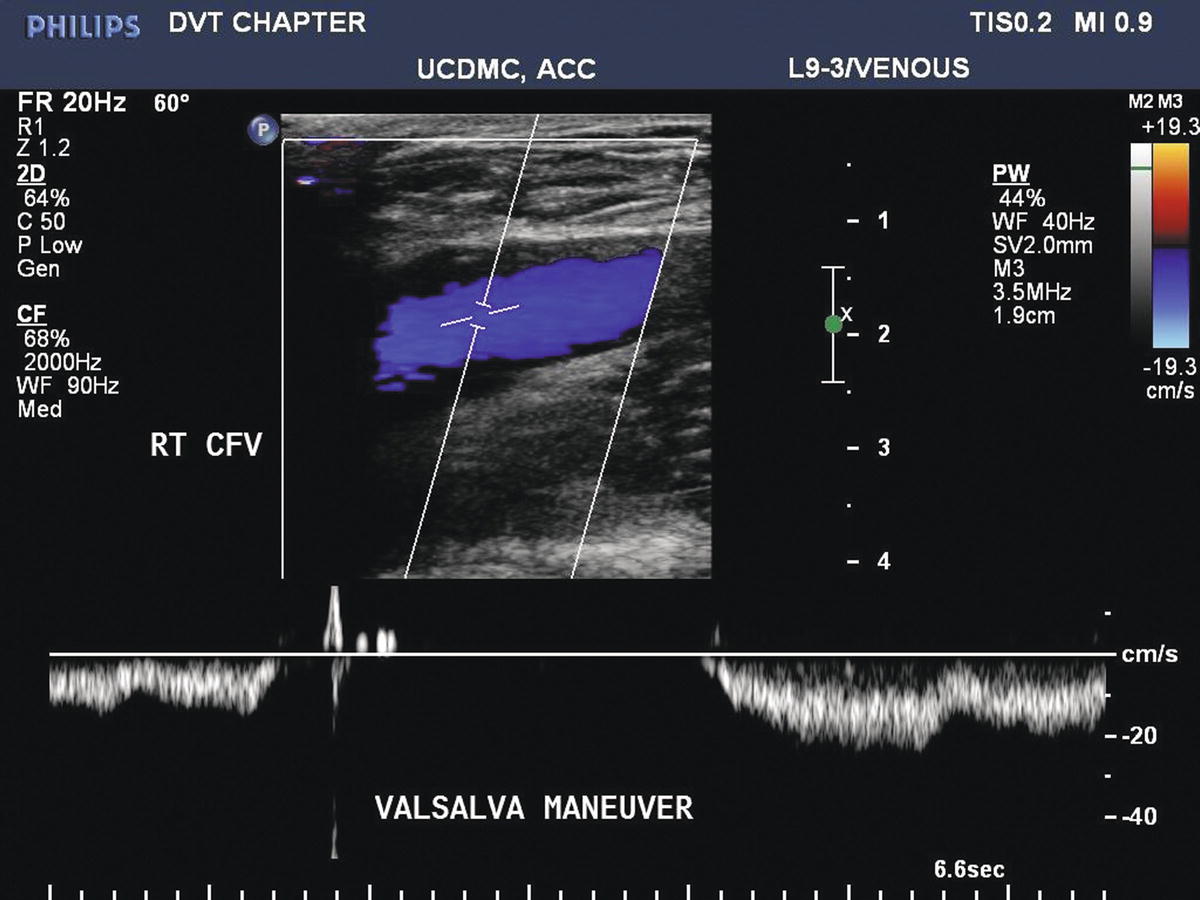
FIGURE 19.10 The Valsalva maneuver increases intra-abdominal pressure and causes antegrade flow to cease in the common femoral vein (CFV). With competent valves, only a very brief period (<0.5 seconds) of retrograde flow is seen before valve closure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


